Background
The introduction of mechanical ventilation for the management of premature infants with severe respiratory distress syndrome in the 1960s changed the natural course of the disease, resulting in increased survival of smaller and sicker infants, many of whom continue to have severe chronic lung damage. Northway and associates were the first to describe this condition in 1967 and introduced the term Bronchopulmonary dysplasia.
Northway's original definition has been extensively modified over the last 4 decades. Bancalari's definition involves ventilation criteria, oxygen requirement at 28 days to maintain arterial oxygen tensions >50 mm Hg, and abnormal findings on chest radiographs. In 1988, Shennan et al proposed that an additional need for supplemental oxygenation at 36 weeks' postmenstrual age may be the most accurate indicator of pulmonary outcome; this criterion decreased the large number of relatively healthy preterm infants Bancalari and others included in their definitions.
All infants in this original description were born prematurely, had severe RDS , and received prolonged mechanical ventilation with high airway pressures and fraction of inspired oxygen ( Fio2). Their clinical and radiographic course ended with severe chronic lung changes characterized by persistent respiratory failure with hypoxemia and hypercapnia, and a chest radiograph that revealed areas of increased density due to fibrosis and collapse surrounded by areas of marked hyperinflation .
Currently, this severe form of chronic lung disease is less common and has been replaced by a milder form of chronic lung damage that occurs in many very small preterm infants who , with increasing frequency , are surviving after prolonged periods of mechanical ventilation . this milder form of lung damage often occurs in infants who initially have only mild pulmonary disease and do not require high airway pressures or fio2. .
Definition Of BPD
There is a striking lack of uniformity in the diagnostic criteria for BPD among clinicians and in the literature. This explains, in part, the wide variation in the reported incidence of BPD among different centers.
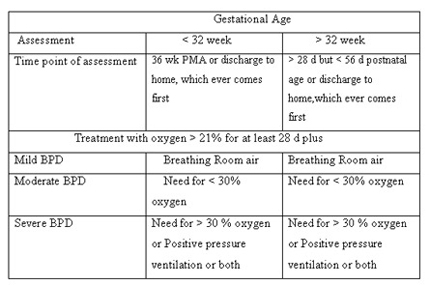
The proposed criteria to define BPD suggested in a National Institutes of Health (NIH) sponsored workshop in 1979 included a continued oxygen dependency during the first 28 days plus compatible clinical and radiographic changes. Although these criteria were appropriate for the classic presentation of BPD, they are not always appropriate for the "new BPD."
In 2001 , a work-shop conducted by the National Institutes of Health proposed a definition that divides BPD in to three categories based on duration and level of oxygen therapy required.
Incidence
Varies by definition, selection bias, survival
Developed countries: NICHD Neonatal Network for 2001
BPD-36 UAB All centers
401-1500g 11% (n=297) 23% (n=3589)
401-1000g 19% (n=154) 39% (n=1517)
Developing countries:
PGI: BPD-28: < 1000g: 50% ; 1000-1249g: 8%; 1250-1499g: 2.3% (Indian Pediatrics Feb 2002)
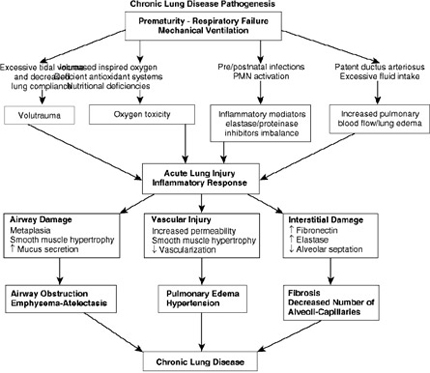
Etiopathogenesis
Factors contributing to development of BPD
In utero
Cytokines (infection)
Direct cause
Mechanical ventilation
O2 toxicity
Baro-Volutrauma
Contributing factors
Prematurity
Primary lung disease
Surfactant deficiency / Dysfunction
Vascular Maldevelopment
family history
Undernutrition
Vitamin A, Vitamin E
Pulmonary edema – PDA, excess fluid load
Infecion (ureaplasma urealyticum)
Antioxidant hypoactivity(o2 radicals)
Poorly inhibited protease activity
Prematurity
Prevalence of CLD in mechanically ventilated infants is inversely related to gestational age and birth weight , strongly suggesting that incomplete development of lungs plays an important role in Pathogenesis of CLD
Oxygen Toxicity and BPD
Oxygen exposure was one of the inciting conditions mentioned in the first description of BPD. Continued exposure to High inspired oxygen levels is accompanied by influx of PMN leucocytes containing proteolytic enzymes .In addition , the antiprotease defense system is significantly impaired in infants exposed to prolonged high inspired oxygen levels , favoring proteolytic damage of structural elements in alveolar walls .
Free radicals can react with intracellular constituents and membrane lipids , thus initiating chain reactions that can cause tissue destruction .
Antioxidant enzymes such as SOD , catalase , and glutathione peroxidase seem to play an important role in preventing the toxic effects of oxygen . Other elements such as Vitamin E, Glutathione and selenium are also part of endogenous antioxidant mechanisms4
A comparison between British centers that used supplemental oxygen sufficient to keep infants’ pulse oximetry values between 88% and 98% and those using target levels of 70% to 90% showed that, among infants born at _28 weeks of gestation, the incidence of continued supplemental oxygen use at 36 weeks PMA was 48% in “high target” centers and 18% in “low target” centers
Mechanical Forces and BPD: Surfactant and Ventilation
Mechanical forces are constantly at work in the lung. Surfactant, a naturally occurring agent that reduces surface tension and minimizes alveolar collapse, is deficient in premature infants with RDS. Although surfactant replacement therapy is beneficial in minimizing symptoms and improving survival in infants with RDS, the efficacy of surfactant replacement in decreasing subsequent BPD incidence and severity is less well-established.
Baro- Volutrauma
High inspiratory pressure has long been identified as a major cause of BPD( barotrauma ) , but tissue damage now is attributed to stretch imposed by excessive alveolar volume ( volutrauma ) . Injury imposed by ventilator leads to alveolar capillary leak and seepage of fluid , plasma and blood in to airways , alveoli and interstitial tissue .Preexisting( intrauterine) inflammation and premature birth enhance vulnerability to ventilator injury.
Atelectatic trauma and volutrauma synergically contribute to pulmonary damage.
Nowadays, it is known that insufficient PEEP (predisposing to atelectatic trauma) and the use of large tidal volumes are the major causes of acute lung injury induced by mechanical ventilation.
Vascular
Maldevelopment in BPD
Coordinated development of the distal epithelium and capillary network is essential to normal lung morphogenesis. BPD may alter both the size and the morphology of the distal capillary bed, affecting lung development and function. In the course of normal fetal lung development, the distal capillary bed expands greatly and undergoes extensive remodeling. Capillary surface area increases eightfold in the last 20% of gestation.
VEGF is a survival factor for endothelial cells and withdrawal results in endothelial death, particularly for nascent endothelial networks. The two main VEGF receptors, VEGFR1 and VEGFR2, are also required for vasculogenesis. Lung VEGF mRNA increases during gestation, mainly in distal airspace epithelial cells, suggesting a critical role for the terminal epithelium in microvascular development.
Disrupted microvascular development and decreased VEGF expression have been associated with BPD in human infants.Distal capillaries were more distant from the epithelial surface than normal, suggestingfailure of remodeling.
Endothelial protein content was also reduced in patients dying with BPD. VEGF andVEGFR1 were reduced in lungs of premature infants dying with BPD and in tracheal aspirates from patients who eventually developed BPD.
Inflammatory Response : Infections
Proinflammatory cytokines , including IL-1, TNF –alfa, IL-8 , IL-6 , PAF ,Prostagladins, leukotrienes and others may be significant in onset of labour .They also have been proposed as likely etiologic factors in the fetal onset of lung injury .
Chorioamnionitis is common in pregnancies that result in premature delivery, being detected in up to 87% of placentas delivered before 27 weeks of gestation. Such placental inflammation has been associated with an increased risk of BPD. That chorioamnionitis may act to mature the lung but also to prime the fetal lung for chronic inflammation Cord blood soluble E-selectin elevation was also predictive of BPD; higher levels correlated with more severe BPD. Genital mycoplasmas, primarily Mycoplasma hominis and Ureaplasma urealyticum, are associated with preterm delivery and are capable of colonizing and stimulating inflammation in the lower respiratory system. Ureaplasma species are the most common organisms isolated from amniotic fluid or placentas of women in pre term labor, with or without prolonged membrane rupture. Choramnionitis with Ureaplasma was associated with elevated amniotic fluid IL-6, IL-1_, TNF-_, and IL-8and increased IL-6 and IL-8 levels in cord blood, as well as with abnormal radiographs and histology suggesting pneumonia.
Fluid Management and PDA
Pulmonary edema is commonly seen in infants with both early and established BPD.Both high volumes of fluid administration and symptomatic PDA have been associated with the later development of BPD.
PDA causes an increase in pulmonary blood flow and interstitial edema, reducing lung compliance and increasing airway resistance. This may result in a more aggressive and prolonged ventilatory strategy, elevating the risk for the development of BPD. Ehrenkranz and mercurio reviewed and subjected to meta- analyses a total of 11 studies that sought closure of ductus arteriosus , either prophylactically or therapeutically. Their Metanalysis did not support the hypothesis that early closure of patent ductus arteriosus diminishes the incidence of BPD.
There is no firm evidence supporting the contention that use of maintenance fluid in high volume contributes to the development of BPD .A Cochrane Review of water intake and its relation to morbidity and mortality in four studies indicated that restricted water intake significantly reduces risks of PDA , NEC and death ; however , there was no significant reduction in the risk for BPD .
Nutrition
Vitamin A
Vitamin A is essential for growth and differentiation of epithelial cells. Vitamin A deficiency is associated with impaired clearance of secretions in the lung, interruption of normal water homeostasis across tracheobronchial epithelium, loss of cilia, diminished ability to repair lung injury, and lack of airway distensability. Extremely LBW (ELBW) infants are deficient in serum and tissue concentrations of retinol. It has been well documented that low plasma retinol levels increased the risk of developing BPD and were associated with long-term respiratory morbidity.
The NICHD Neonatal Research Networkmulticenter randomized controlled trial involving 807 ELBW infants at risk demonstrated a significant decrease inthe incidence of BPD or death in the vitamin A-treated group.Further, the study found trends toward reduced risk of sepsis in the vitamin A-treated group.
Vitamin E
Vitamin E is an antioxidant that protects cell membranes from oxidative injury. Deficiency of vitamin E worsens the effect of oxygen toxicity to lung tissue.
Metaanalysis of eight RCT demonstrated that supplemental vit E doesnot diminish the incidence of BPD.
Inositol
Inositol is a naturally occurring nutrient involved in phospholipids which functions as a cellular mediator of signal transduction, metabolic regulation, and growth. It promotes endothelial cell growth, enhances glucocorticoid-mediated lung epithelial cell differentiation, and may serve as an antioxidant.
It plays a critical role in surfactant synthesis. Metaanalysis of infants supplemented with inositol demonstrated statistically significant reduction in death or BPD
PATHOLOGY
The classic BPD was characterized by severe morphologic changes that included emphysema, atelectasis and fibrosis, and marked epithelial squamous metaplasia and smooth muscle hypertrophy in the airways and in the pulmonary vasculature. These changes were associated with severe respiratory
failure with airway obstruction, pulmonary hypertension, and cor pulmonale. The milder form of BPD seen more frequently today is characterized mainly by increased lung fluid, a diffuse inflammatory response, and a striking decrease in alveolar septation and impaired vascular development.
Time for clinical onset of BPD is difficult to designate
In usual circumstances , at approximately 7 days after birth ,an neonate being treated for RDS has not improved as expected & diminished ventilator support is not feasible .
OR improvement has occurred & ventilatory support is decreased serially or discontinued ,but soon thereafter an abrupt need for increased O2 supplementation & ventilatory pressures arises. In these circumstance, protracted treatment for CLD has begun .
Develop through 4 stages
Acute RDS (1- 3 days)
Period of lung regeneration (4 -10 days)
Period of transtition to chronic disease(10-20 days)
Chronic disease (30 days)
Now most BPD are mild to moderate ,characterized by an initial need for mechanical ventilatory support longer than initially anticipated.
This prolong support is followed by days or weeks of O2 supplementation in a hood .
Mild BPD , radiologic indication often is uncertain ,even though retractions , generally diminished breath sounds , & crepitant rales are prominent
Moderate & severe disease associated with radiographic appearance that highly suggestive of BPD.
O2 conc & ventilatory pressures must be increased relentlessly.
Clinically barrel chest
As a rule, if respiratory support can be diminished in an infant at any time within first month of life– course of BPD is relatively benign.
Need for increased support at about this time often progress to severe protracted disease.
Acute episodes of respiratory deterioration often occur at any time during the course of disease , whether or not infant is on mechanical ventilation .
One must consider :
1.Infection
2.Pulmonary edema
? PDA
? Overhydration
? progression of chronic disease
3.CCF
4.Bronchospasm
Bronchospasm : Usually temporary increase in PIP by 2 or 3 with an increase of FiO2 to 1.0 totally eliminate bronchospasm with out need of pharmacologic management.
Ineffective after 2 min ,bronchodilating agents mandatory.
Infant older than 1 month , progressive BPD –right sided heart failure / RVH universal.( resistance to pulmonary blood flow). Growth failure is prominent (require 25% more calories than unaffected infants) Osteopenia is common extreme premature babies.
Low calcium & Vitamin D intake
diuretic therapy – significant calciuria
Fractures of long bones & ribs – routine nursing care –unexpected findings on radiography
Extubation is followed by capricious respiratory course.
1.Inspiratory stridor – tracheal edema, scarring / both evident immediately after extubation.
2.Tracheal stenosis – several days to weeks.
3.Severe episodes of wheezing – bronchospasm
4.O2 requirements wax & wane
5.Overproduction of secretions .
6.Survival beyond 7 or 8 months – increased likely hood of normal cardiopulmonary function by 5 to 6 yrs .
Systemic HTN- benign & amenable to appropriate therapy
Clinical risk factors for hypertension included a high incidence of bronchodilator therapy and use of diuretics , as well as need for longer course of O2 therapy at home.
?Later Months & Beyond
Principal hazards
1.SIDS
2.Recurrent respiratory infection (RSV)
3.Acute episodes of bronchospasm
4.Aspiration during feedings
?Growth is retarded & doesnot assume a normal rate until after the child is 2-5 yrs .
?With improved respiratory status beyond 2 yrs of age ,growth in height & HC is accelerated, as is weight gain but to a lesser degree.
?Developmental delays
Respiratory infection is by far the most frequent cause of readmission . Recurrent illness is reported in 50% to 85% of patients. Other causes of readmission are bronchospasm , corpulmonale , upper airway obstruction , surgery, systemic hypertension , & family social crises.
NEW BPD
?Milder clinical disease.
?Beneficial consequence of improved early management.
?X-ray typically hazy throughout the lungs , associated with variable degrees of diffuse hyperinflation.
?Blood gases are moderately abnormal
?Episodes of hypoxemia & hypercarbia are common.
?Wheeze & acute episodes of pulmonary edema are recurrent ( not severe)
?Feeding difficulties & growth retardation are not profund
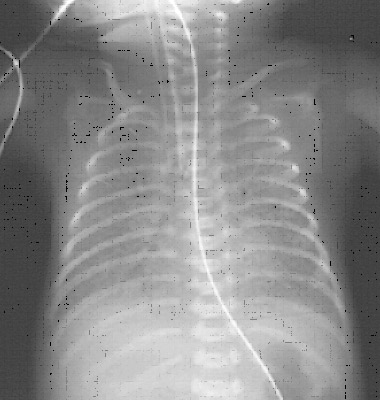 Radiologic characteristics
Radiologic characteristics
1. Stage 1(mild BPD):identical to RDS
Diffuse reticulogranularity & air bronchograms
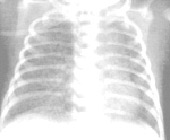
2. Stage 2 (Moderate BPD):
? Virtually homogenous opacification of lungs that obscures cardiac margins .
?Early radiographic changes seen in mild disease – replaced by coarse , irregularly shaped densities that are confluent & may contain vacuolar radioluciencies.
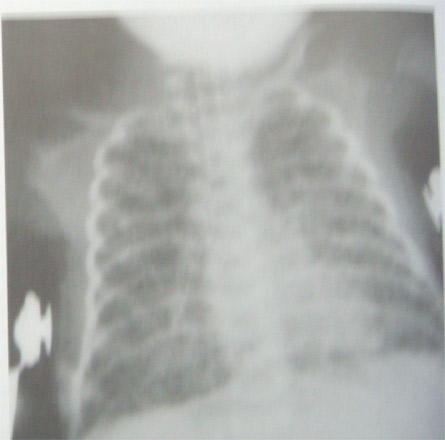
4. Stage 4( Advanced disease) :
Lungs appear bubbly on radiography as air cysts or continue to enlarge . Opacities are reduced to strands , streaks & small patches as cysts expand.
MANAGEMENT
Ventilatory Strategies in the Prevention and management of Bronchopulmonary Dysplasia
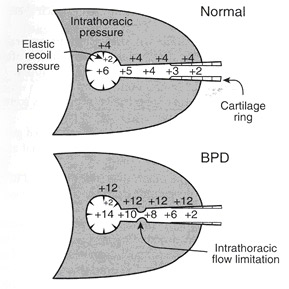
In general, the ventilatory strategies to prevent and manage BPD are based on reducing the magnitude and duration of mechanical ventilatory support to the minimum possible while achieving adequate gas exchange. By two main strategies:
(1) redefining the goals for “adequate gas exchange” leading to reduced use of mechanical ventilation support
• Targeting Higher PaCO2 ( Permissive Hypercapnia)
• Tolerating a Lower SpO2(Permissive Hypoxemia)
(2) refining the methods of mechanical ventilation or using alternative techniques.
• Lower Pressures, Faster Rates, Shorter Inspiratory Times (Ti)
• Volume – Targetted Ventilation
• Patient – Triggered Ventilation
Suggested Ventilator Settings and Targets for Infants with Early RDS, Evolving BPD, and Established BPD
Clinical Scenario Ventilator Settings and Strategies Targets
RDS (< 1 week age) 1. Early therapeutic CPAP SpO2 87-92%
2. Early surfactant pH
7.25-7.35
3. Methylxanthines/Vitamin A if indicated PaO2 40-60 mm Hg
4. Rapid rates (40-60/min) PaCO2 45-55 mm Hg
5. Moderate PEEP (4-5 cm H2O)
6. Low PIP (10-20 cm H2O)
7. Short Ti (0.25-0.4 s)
8. Low tidal volume (3-6 mL/kg)
9. Early extubation to NCPAP/SNIPPV
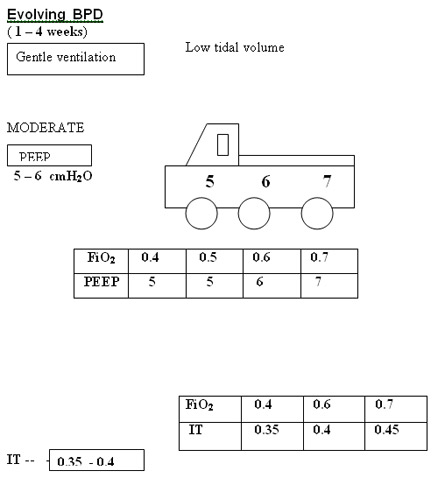
Evolving BPD (1–4 weeks age) 1. Methylxanthines SpO2 88-93%
2. Slower rates (25-40/min) pH 7.25-7.35
3. Moderate PEEP (4-5 cm H2O) PaO2 50-70 mm Hg
4. Low PIP (10-20 cm H2O) PaCO2 50-60 mm Hg
5. Moderate Ti (0.35-0.45 s)
6. Low tidal volume (3-6 mL/kg)
7. Early extubation to NCPAP/SNIPPV
Established BPD (>4 weeks age) 1. Slow rates (20-40/min) SpO2 89-94%
2. Moderate PEEP (4-8 cm H2O) pH 7.25-7.30
3. Lowest PIP required (20-30cm H2O) PaO2 50-70 mm Hg
4. Longer Ti (0.4-0.7 s) PaCO2 55-65 mm Hg
5. Slightly larger tidal volume (5-8 mL/kg)
BRONCHOPULMONARY DYSPLASIA
RDS < 1 week
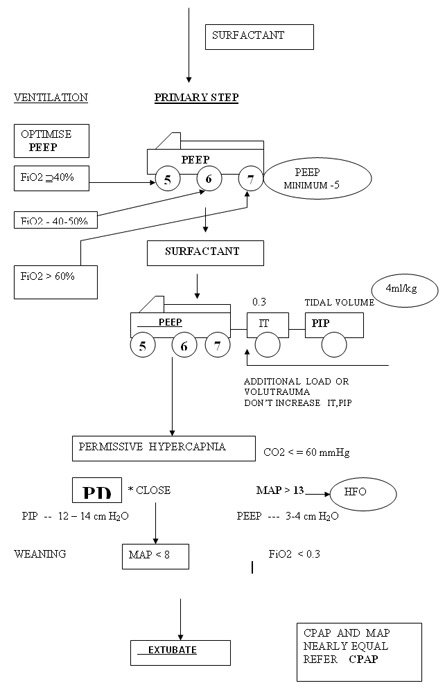
Don’t change PIP or Tidal volume to change MAP and Oxygenation. Peep is the only variable to be adjusted in RDS based on Chest X-ray and FiO2 . Tidal volume at 4 ml/kg will guide the beginner to optimise PIP and chest movements. Don’t change IT to effect Oxygenation by increasing MAP. Increasing IT and PIP to increase MAP may the primary step in the genesis of BPD. Accept higher PaCO2 initiallt >= 70 mmHg and observe a sequential steady fall in CO2 as the lung improves if the tidal volume and hence PIP are right.
KEY WORDS IN THE MANAGEMENT OF BPD
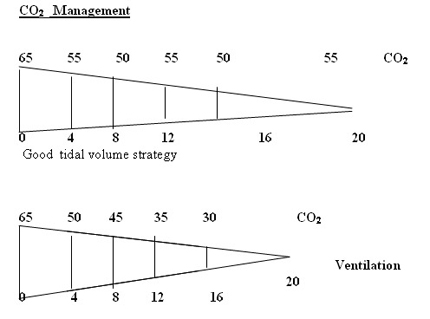
1. Accept a wide range of PaCO2 55 – 75 mmHg (Gentle ventilation)
2. Don’t increase PIP , IT inadvertently to increase MAP. IT 0.3 – 0.35 secs is reasonable for all atelectatic disease. 4 ml/kg tidal volume will optimise the setting of PIP. Don’t increase to 5 ml/kg to effect oxygenation.
3. PEEP is the primary determinant of Oxygenation “Higher PEEP” is advisable to lower PEEP.
4. Early extubation to CPAP when MAP is near 8.
5. If MAP is more than 13 or PIP is more than 25 cm H2O , one may consider HFO.
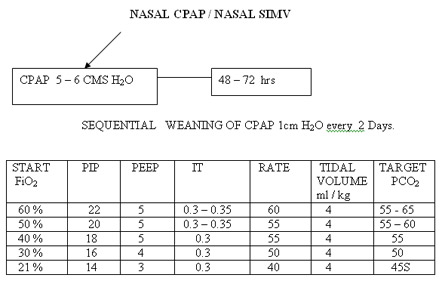
6. Sequential weaning off CPAP is the preferred practice in our unit compared to abrupt weaning.
Pathological Consideration -

Pathological Consideration
PIP guided by TV 4 ml/kg to obtain reasonable and excessive chest movement.
PaCO2 >= 55 – 65 mmHg.
If MAP reaches close to 8 attempt to extubate to CPAP.
Established BPD ( > 4 weeks)
• AIR TRAPPING DISEASE
• + • PEEP
• IT , TV in hypoxic spells
• Tracheostomy
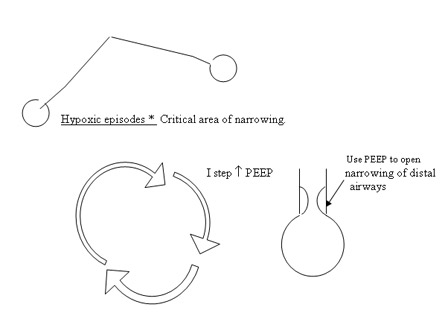
PEEP appears to be critical in maintaining distal airways from collapse. In severe BPD , PEEP upto 9 cm H2O may be required to get adequate gas exchange and reduce hypoxic spells (Fig ). Hogher PEEPs have been used in some units in the review of literature (upto 10 – 12 cmH2O).


Longer IT have been used in the review literarure but in our unit IT between 0.4 to 0.5 seems to be adequate even in severe BPD . Slow rates have to used to give sufficient expiratory time .
HYPOXIC SPELS
The primary area involved is the distal airways which are being remodelled and hence prone to collapse easily. An addition of extra PEEP 0.5 – 1 cmH2O stabilizes these airways . Review literature maintains that increasing the tidal volume by
0.5 – 1 ml/kg be in the primary step to reduce these episodes. Our experience points to increasing PEEP is a primary manoeuvre followed by an increment in the tidal volume to 5 ml/kg. IT is not to be changed randomly in these situations. In all strategies at this present time in BPD , gentle ventilation I(CO2 >= 55 – 60 mmHg) and lower rates are preferred to reduce further progression of BPD. Hypoxemia is to be prevented at all costs and PaO2 should be more than 50 mmHg to prevent exacerbation of pulmonary hypertension . Larger tidal volumes though recommended are not uniformly applicable since they could aggravate volutrauma . Reduction in PEEP by 0.5 – 1 cmH2O would be advised once in 3 – 5 days depending on the frequency of hypoxic spells.
Tracheostomy offers a better broncho-pulmonary hygiene and should be considered if the babies fail extubation twice. Babies are managed betterb from airway stand point and reducion in airway pressures , PIP ,PEEP would also be evidenced after tracheostomy. Time to discharge is also facilitated by this procedure. The frequency of hypoxic spells are also noted to be decreased.
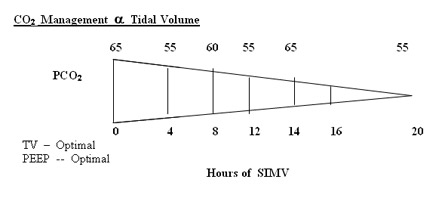
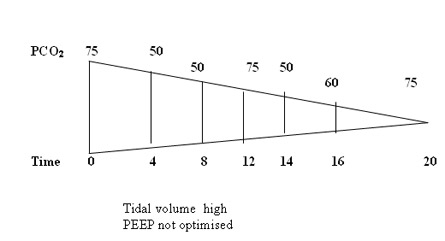
In our unit we rarely use TV above 5 ml/kg , generally managed with TV of 4 m/kg and titrating PEEP levels and X-rays to reduce hypoxic spells . Volume targeted ventilation appears promising and may be considered ideal but in BPD the leak registered on the flow sensor make it clinically difficult to use volume during bronchospasm . Pressure ventilation to get reasonable chest movement is desired and once the air leak on the flow sensor reduces to 30% the tidal volume estimation can be used to guide titrate the PIP.
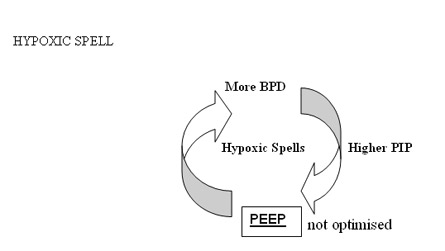
BPD Zone --- Conceptual models to reduce BPD
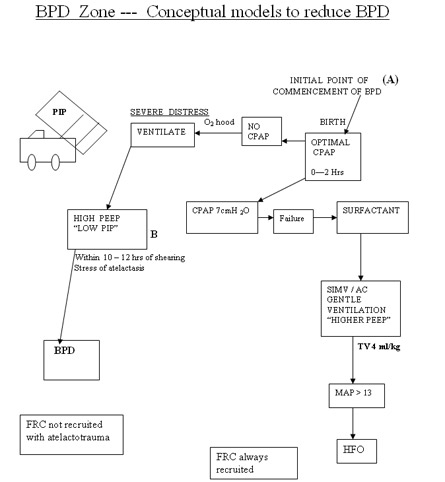
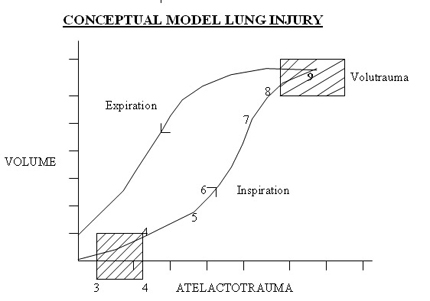
Low PEEP and high PIP to achieve higher MAP to increase oxygenation is the first wrong steps in the genesis of BPD. Setting optimum PEEP is the basic in the genesis of BPD. "Higher" PEEP by 1 cmH2O compared to our earlier understanding of PEEP may be beneficial in most cases of lung diseases. It is mandatory to monitor tidal volume at 4 ml/kg to reduce the incidence of BPD. Any tidal volume in excess of 6 ml/kg would predisposes the lung to shearing stress and BPD . A blood CO2 between 55 - 65 mmHg would generally be gentle to the lung by reducing volutrauma. On all the mechanical ventilators PIP controls tidal volume. If the tidal volume is optimal rate should be optimised to keep CO2 at above levels. Shearing stress of disproportionate Tidal Volume facilitates the development of BPD. The deflation-inflation sequence is primary to the capillary leakage and cytokine release in the alveoli.
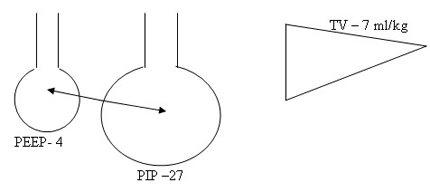
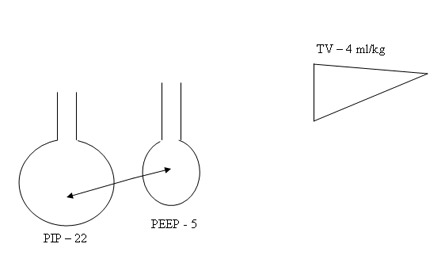
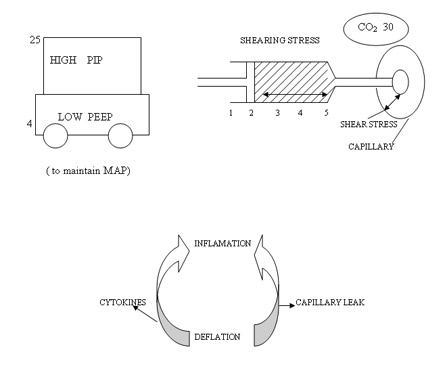
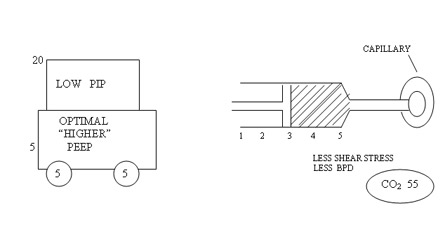
Pharmacological Strategies in the Prevention and Management of Bronchopulmonary Dysplasia
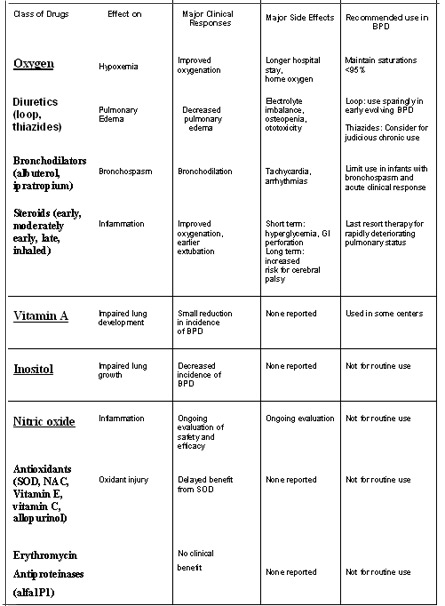
Oxygen
Judicial use of oxygen to maintain oxygen saturations and pO2 levels within target range is important in preventing pulmonary hypertension and cor pulmonale in infants with BPD. Although there is general acceptance that oxygen should be used and monitored as a medication with strict guidelines, there is no agreement on the acceptable level of oxygen saturation below which oxygen should be administered to preterm infants at risk for BPD or to maintain oxygenation in infants with established BPD.37 Askie and colleagues showed in a double-blind randomized trial (BOOST) that there is no benefit in maintaining high oxygen saturation (95% to 98%) compared with standard (91% to 94%) oxygen saturation level in preterm infants.38
Diuretics
Diuretics constitute one of the most common classes of drugs used in the management of BPD. BPD in premature infants is complicated by interstitial alveolar edema. The two most common diuretics used in BPD, loop diuretics and thiazides, differ in their primary site of action on the nephron.
Loop Diuretics
Loop diuretics act principally by blocking the luminal Na-K- 2Cl transporter in the thick ascending limb of the loop of Henle. Loop diuretics may improve pulmonary mechanics in patients with lung edema through both diuretic-independent and diuretic-dependent responses.
The diuretic-independent response is of immediate onset and occurs through cyclooxygenase-mediated pulmonary vasodilation, decrease in the transpulmonary fluid filtration, increase in interstitial fluid reabsorption, and systemic vasodilation that decreases pulmonary fluid load.
Later, thediuretic-dependent response reduces extracellular volume by an increase in the urine output, which, along with higher capacitance of the systemic vessels, decreases filling pressureof the left heart and pulmonary capillaries. Side effects include Hypercalciuria, nephrocalcinisis, ototoxicity, electrolyte imbalance , and nephrolithiasis Dosage : 1-2mg/kg every 12 hrly
Thiazides
The second group of diuretics localizes its effects to the early portion of the distal tubule. Thiazides are the diuretics of choice in ventilator-dependent infants with evolving or established BPD. It decreases calcium excretion.
Dosage 10- 20mg/kg every 12 hrly
More importantly, adding spironolactone did not decrease the risk for sodium or potassium supplementation in patients receiving thiazides.
Spironolactone 0.5- 1.5 mg/kg every 12 hr
Bronchodilators
Patients with BPD have increased airway resistance due to smooth muscle hypertrophy and hyperreactivity. Bronchodilators have been broadly categorized into adrenergic and anticholinergic agents. Their effect is transient and both have been shown to acutely reduce pulmonary resistance and increase compliance in BPD patients.
The two most widely used bronchodilators are albuterol and ipratropium.
Potential side effects of Beta sympathomimetic agents include tachycardia, hypokalemia, arrhythmias, and hyperglycemia. Inhaled anticholinergic agents, in addition, decrease gastrointestinal motility and dry and thicken respiratory secretions.
Dosage : Albuterol ( inhaled) 0.1-0.5mg/kg every 2-6 hr
Albuterol (oral) 0.1- 0.5 mg /kg every 6-8 hr
Theophylline(oral) 5-6 mg/kg, loading dose , 1-2mg/kg every 8-12 hr ,
Maintenance dose
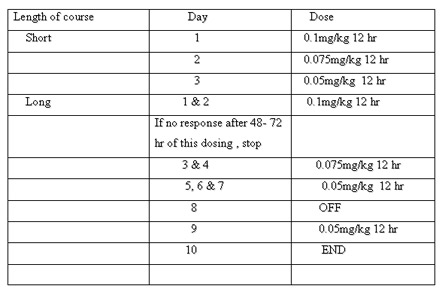
Corticosteroids
Inflammation is a main contributor to the pathogenesis of BPD. Systemic steroid administration reduces the inflammatory response, and also enhance surfactant production, decrease airway edema, stabilize capillaryleakage, augment _ adrenergic activity, and decrease overall lung fibrosis. and thus produces a rapid improvementin pulmonary function with better gas exchange, and facilitates weaning from mechanical ventilation.
Postnatal steroids have been used either for prevention of CLD or to treat it once established. Based on the timing of initiation of the treatment, it can be categorized into 3 broad groups as follows:
(i) Early treatment, during the first 96 hrs after birth;
(ii) Moderately early treatment, between postnatal days 7 and 14days ;
(iii) Delayed treatment, given after 3 weeks of age
The Cochrane Neonatal Review Group (CNRG), which provides the most valid and reliable evidence on which to base decisions concerning treatment, has published 3 meta-analyses by incorporating all the controlled trials of postnatal steroids for CLD
a) Beneficial outcomes
All the three regimes significantly reduce the incidence of combined outcome of death or CLD at 36 weeks PMA and also facilitate early extubation by 7th day after commencing steroid treatment.
While both early and moderately early steroid therapy reduce the incidence of CLD at 36weeks PMA, late steroid therapy reduces the need for home oxygen therapy. However, they do not have significant effect on mortality alone. b)Short-term side effects
Infection, hyperglycemia, hypertension and GI bleeding are either significantly increased or show a trend towards increased incidence with early and moderately early steroid therapy .
Growth failure is seen with both early and late steroid therapy. The incidences of severe intraventricular hemorrhage (IVH), periventricular leucomalacia (PVL) and necrotizing enterocolitis (NEC) are not significantly increased with steroid therapy.
c) Long-term adverse effects
Barrington KJ performed a systematic review by identifying 3 additional trials that reported on the long term outcome after postnatal steroid treatment
The infants who received PNS were twice as likely to develop cerebral palsy as the control infants. Similarly, steroid treated infants have 1.34 times the risk of having neurodevelopmental impairment.
The mechanisms by which steroids affect brain growth remain unclear, but the possible theories are
(a) inhibition of growth factors and facilitation of apoptosis
(b) by modifying the properties of NMDA glutamate receptors that lead to neuronal cell death following the release of glutamate (c) due to the effect of neurotoxic sulphite preservatives present in injectable preparations of dexamethasone. Sulphites have
excitotoxic like properties and may play a key role in steroid induced brain damage. It is interesting to note that injectable betamethasone does not contain this preservative.
AAP/CPS statement
Pediatrics 109: 330-8 Feb 2002
"The routine use of systemic dexamethasone for the prevention or treatment of chronic lung disease in infants with very low birth weight is not recommended"
"Outside the context of a randomized, controlled trial, the use of corticosteroids should be limited to exceptional clinical circumstances (eg, an infant on maximal ventilatory and oxygen support)."
Vitamin A
After studying variousregimens for dosing and checking serum retinol concentrations, the standard dose of 5000 IU IM per dose given 3times per week for 4 weeks (12 doses) has been recommended for the infants at risk.
Our current recommendations are to give IM vitamin A three times a week for 12 total doses to infants with birth weight less than 1000 g.
Nutrition :Providing nutrition to premature with BPD varies little from prematures without BPD.
1.Need more calories
2.Increments in calorie intake imply an increased fluid volumeÓ BPD may not tolerate Ó propensity to develop pulmonary edema
3.Formulas of increased calorie density (up to 30kcal/oz) Ó satisfy required calories with relatively low fluid volume
Caloric Demands (Kcal/kg/day) of Infants with BPD*
Demand Acute Transition Chronic
Basal metabolic rate 40 60 60
Thermal stress 0-5 0-10 10
Activity 0-5 5-15 10-30
Stool losses 0-10 5-10 10
Specific dynamic action 0 0-5 10
Growth 0 20-30 20-30_
Total 40-60 90-130 120-150
*(Adapted from Niermeyer S: Nutritional and metabolic problems in
infants with bronchopulmonary dysplasia)
Theseinfants should receive additional calcium, phosphorus, andvitamin D supplementation through premature formula or fortification of breast milk.
Recommendations for Parenteral Nutritional Management of Hospitalized VLBW Infants
Transitional Period
Initiation Increments
Maintenance Period
Fluids (ml/kg/day) 80 to 100 10 to 20 120-150
Calories (Kcal/kg/day) 40 to 60 10 to 15 120
Carbohydrate (Glucose infusion rate)
(mg/kg/min) 4 to 6 1
10 to 12 Protein (Amino acids) (gm/kg/day) 3 0.5 to 1 4
Lipids (gm/kg/day) 0.5 to 1 0.5 to 1 3
Sodium (meq/kg/day) Minimal (first several days) 2 (4 to 6 days of life) 3 (after day 6)
Max fluid vol 140-150ml/kg/day
Protein 3.5g/kg/d
Enriched formula (30cal/oz, 1.0kcal/ml)
Vitamin A 5000IU IM, 3 times weekly for 4 weeks
Prevention of BPD
Ventilatory Strategies
Selective intubation / Avoid IMV (Prophylactic IMV bad)
Early CPAP
Minimal ('gentle") ventilation
Early extubation
Pharmacologic Strategies
Antenatal steroids
Vitamin A supplementation
Others
Other management: PDA, Infection
Bibliography
1. Northway WH, et al : Pulmonary disease following respirator therapy of hyaline membrane disease : Bronchopulmonary dysplasia. N Engl J Med 323:1793,1990.
2. Rojas M, et al : Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease .J pediatr 126:605, 1995.
3. Bancalari E, Abdenour GE, Feller R, et al: Bronchopulmonary dysplasia: clinical presentation. J Pediatr 95:819-823, 1979.
4. Fanaroff, Martin :Neonatal Chronic Lung disease :Neonatal -Perinatal Medicine, Diseases of the fetus and infant 8th edition vol2:1155-1168,2006.
5. Tin W, Milligan DW, Pennefather P, Hey E: Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonatal Ed 84:F106-F110, 2001.
6. Reynolds EO, Roberton NR, Wigglesworth JS: Hyaline membrane disease, respiratory distress, and surfactant deficiency. Pediatrics 42:758-768, 1968.
7. Kendig JW, Notter RH, Cox C, et al: A comparison of surfactant as immediate prophylaxis and as rescue therapy in newborns of less than 30 weeks' gestation. N Engl J Med 324:865-871, 1991.
8. Bancalari E, del Moral T: Bronchopulmonary dysplasia and surfactant.Biol Neonate 80:7-13, 2001 (suppl 1)
9. AutenRL, Vozzelli M, ClarkRH : Volutrauma.What is it ,and how do we avoid it ? Clin Perinatol 28:505, 2001.
10. Dreyfuss D, saumon G : Barotrauma is volutrauma, but which volume is the one responsible ?( editorial). Intensive care med 18:139, 1992.
11. Moschopulos M, Burri PH: Morphometric analysis of fetal rat lung development. Anat Rec 237:38-48, 1993
12. Gerber HP, McMurtrey A, Kowalski J, et al: Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3=-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273:30336-30343, 1998.
13. Alon T, Hemo I, Itin A, et al: Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1:1024-1028, 1995.
14. Bhatt AJ, Amin SB, Chess PR, et al: Expression of vascular endothelial growth factor and Flk-1 in developing and glucocorticoid-treated mouse lung. Pediatr Res 47:606-613, 2000
15. Coalson J: Pathology of chronic lung disease in early infancy, in BlandR, Coalson J (eds): Chronic Lung Disease in Early Infancy (vol. 137). New York, NY,Marcel Dekker, 2000, pp 85-124.
16. Bhatt AJ, Pryhuber GS, Huyck H, et al: Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 164:1971-1980, 2001. 17. Thibeault DW, Mabry SM, Norberg M, et al: Lung microvascular adaptation in infants with chronic lung disease. Biol Neonate 85:273- 282, 2004.
18. Lassus P, Turanlahti M, Heikkila P, et al: Pulmonary vascular endothelialgrowth factor and Flt-1 in fetuses, in acute and chronic lungdisease, and in persistent pulmonary hypertension of the newborn.Am J Respir Crit Care Med 164:1981-1987, 2001
19. YoonBH, Romero R, Yang SH , et al : Interleukin -6 concentrations in Umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia.Am J Obstet Gynecol 174:1433, 1996.
20. Viscardi RM, Muhumuza CK, Rodriguez A, et al: Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res 55:1009-1017, 2004
21. Baier RJ, Loggins J, Kruger TE: Monocyte chemoattractant protein-1 and interleukin-8 are increased in bronchopulmonary dysplasia: relationto isolation of Ureaplasma urealyticum. J Invest Med 49:362-369, 2001
22. Witt A, Berger A, Gruber CJ, et al: Increased intrauterine frequency of Ureaplasma urealyticum in women with preterm labor and pretermpremature rupture of the membranes and subsequent cesarean delivery.Am J Obstet Gynecol 193:1663-1669, 2005.
23. Gomez R, Ghezzi F, Romero R, et al: Premature labor and intraamniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol 22:281-342, 1995.
24. Witt A, Berger A, Gruber CJ, et al: IL-8 concentrations in maternal serum, amniotic fluid and cord blood in relation to different pathogens within the amniotic cavity. J Perinat Med 33:22-26, 2005.
25. Northway WH Jr: Bronchopulmonary dysplasia: thirty-three yearslater. Pediatr Pulmonol Suppl 23:5-7, 2001
26. Oh W, Poindexter BB, Perritt R, et al: Association between fluid intakeand weight loss during the first ten days of life and risk of bronchopulmonarydysplasia in extremely low birth weight infants. J Pediatr147:786-790, 2005
27. Tammela OK, Koivisto ME: Fluid restriction for preventing Bronchopulmonary dysplasia? Reduced fluid intake during the first weeks oflife improves theoutcome of low-birth-weight infants. Acta Paediatr 81:207-212, 1992
28. Ehrenkranz RA , Mercurio MR : Bronchopulmonary Dysplasia. In Sinclair JC , Bracken MB ( eds) : Effective Care of the newborn infant . Oxford, Oxford University Press, 1992, p.399.
29. Bell EF, Acarregui MJ : restricted versus liberal water intake for preventing morbidity and mortality in preterm infants ( Cochrane review ).Available at : http://www.cochranelibrary .com
30. Spears K, Cheney C, Zerzan J: Low plasma retinol concentrations increasethe risk of developing bronchopulmonary dysplasia and longtermrespiratory disability in very-low-birth-weight infants. Am J ClinNutr 80:1589-1594, 2004.
31. Tyson JE, Wright LL, Oh W, et al: Vitamin A supplementation for extremely-low-birth-weight infants. N Engl J Med 340:1962-1968,1999.
32. Howlett A, Ohlsson A: Inositol for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev 4:CD000366, 2003
33. Merritt TA , Boynton BR : Clinical presentation of Bronchopulmonary dysplasia. In Merritt T A, Northway WH Jr, Boynton BR (eds):Bronchopulmonary dysplasia. Boston, Blackwell Scientific Publications, 1998,pp.179-184.
34. JohnsonV: Editorial : Systemic Hypertension in infants with severe Bronchopulmonary dysplasia. Am J Perinatol 10:260, 1993.
35. Brown ER : Bronchopulmonary dysplasia..In Rubin IL, Crocker AC (eds): Developmental disabilities : Delivery of Medical care for children and adults .Philadelphia,Lea and Febiger, 1989 ,p.223.
36. KoopsBL,Lam C: Outcome in Bronchopulmonary dysplasia: Motality risks and prognosis for growth , neurological integrity , and developmental performance .In Bancalari E,Stocker JT ( eds) : Bronchopulmonary dysplasia.Washington , DC , Hemishere publishing Corporation , !988,p.403.
37. Abman SH, Wolfe RR, Accurso FJ, et al: Pulmonary vascular responseto oxygen in infants with severe bronchopulmonary dysplasia. Pediatrics75:80-84, 1985.
38. Askie LM, Henderson-Smart DJ, Irwig L, et al: Oxygen-saturationtargets and outcomes in extremely preterm infants. N Engl J Med349:959-967, 2003.
39. Brion LP, Primhak RA: Intravenous or enteral loop diuretics for preterminfants with (or developing) chronic lung disease. Cochrane Database Syst Rev CD001453, 2002.
40. Ng GY, da S, Ohlsson A: Bronchodilators for the prevention andtreatment of chronic lung disease in preterm infants. Cochrane Database Syst Rev CD003214, 2001.
41. Davis JM, Sinkin RA, Aranda JV: Drug therapy for Bronchopulmonary dysplasia. Pediatr Pulmonol 8:117-125, 1990.
42. Roberts JM, Jacobs MM, Cheng JB, et al: Fetal pulmonary beta-adrenergicreceptors: characterization in the human and in vitro modulationby glucocorticoids in the rabbit. Pediatr Pulmonol 1:S69-S76,1985.
43. Cole CH, Fiascone JM: Strategies for prevention of neonatal chronic lung disease. Semin Perinatol 24:445-462, 2000.
44. Baud O. Postnatal steroid treatment and braindevelopment. Arch Dis Child F Neonatal edn. 2004;89: F96-F100
45. Halliday HL, Ehrenkranz RA. Early postnatal (< 96hours) corticosteroids for preventing chronic lungdisease in preterm infants. Cochrane Database SystRev 2003; (1): CD001146.
46. Halliday HL, Ehrenkranz RA. Moderately early (7-14days) postnatal corticosteroids for preventing chroniclung disease in preterm infants. Cochrane DatabaseSyst Rev 2003; 1: CD001144.
47. Halliday HL, Ehrenkranz RA. Delayed (.3 weeks)postnatal corticosteroids for chronic lung disease inpreterm infants. Cochrane Database Syst Rev 2003; 1:CD001145.
48. Barrington KJ. The adverse neuro-developmentaleffects of postnatal steroids in the preterm infant: asystematic review of RCTs. BMC Pediatrics 2001; 1:1-14.
49. Jacobs HC, Chapman RL, Gross I. Prematureconclusions on postnatal steroid effects. Pediatrics2002; 110: 200-201.
50. Finer NN, Powers RJ, Chia-hao Simon Ou, Durand D,Wirtschafter D, Gould JB for the California PerinatalQuality Care Collaborative Executive Committee.Prospective Evaluation of Postnatal SteroidAdministration: A 1-Year Experience From theCalifornia Perinatal Quality Care Collaborative.Pediatrics 2006; 117: 704-713.
51. Shinwell ES, Karplus M, Bader D, Dollberg S, Gur I,Weintraub Z, et al. Neonatologists are using much lessdexamethasone. Arch Dis Child Fetal Neonatal Ed2003; 88: F36-F40.
52. Kennedy KA, Stoll BJ, Ehrenkranz RA, et al: Vitamin A to preventbronchopulmonary dysplasia in very-low-birth-weight infants: has thedose been too low? The NICHD Neonatal Research Network. EarlyHum Dev 49:19-31, 1997
53. Ambalavanan N, Wu TJ, Tyson JE, et al: A comparison of three vitaminA dosing regimens in extremely-low-birth-weight infants. J Pediatr142:656-661, 2003
Original Article
Inspiratory time and pulmonary function in mechanically ventilated babies with chronic lung disease Dr. Steven L. Goldman, MD *, Ellen M. McCann, MS, Benjamin W. Lloyd, MD, Gary Yup, MD Department of Pediatrics, California Pacific Medical Center (formerly Children's Hospital of San Francisco and the Cardiovascular Research Institute, University of California, San Francisco, California *Correspondence to Steven L. Goldman, California Pacific Medical Center, Newborn Services, 3850 California Street, San Francisco, CA 94118
Keywords
Tidal volume . dynamic lung compliance . lung resistance . functional residual capacity . anatomical dead space/tidal volume . alveolar ventilation Abstract
To learn if increasing inspiratory time would improve pulmonary function in mechanically ventilated babies with chronic lung disease, we measured lung mechanics and alveolar ventilation at three inspiratory times: 0.4, 0.6, and 0.8 s. Nine babies were studied. Their mean birth weight was 875 g (range, 570-1,100 g), gestational age 27 (24-34) weeks, and age 7 (4-12) weeks. Their mean oxygen requirement was 40% (29-53), ventilator rate 33/min (20-40), and mean airway pressure 8 (5-10) cmH2O. Ventilator rate was kept constant; therefore expiratory time decreased and mean airway pressure and I:E ratio increased at longer inspiratory times. At 0.6 s and 0.8 s, when compared to 0.4 s, significant increases occurred in tidal volume (10.4, 10.1, and 8.4 mL/kg, respectively), dynamic lung compliance (0.68, 0.68, and 0.53 mL/cmH2O/kg, respectively), and alveolar ventilation (6.0, 6.3, and 4.7 mL/kg/breath, respectively). Airway resistance, anatomical dead space to tidal volume ratio, and functional residual capacity were similar at the three inspiratory times. Our findings suggest that an inspiratory time 0.6 s (compared to 0.4 s) increases the effectiveness of mechanical ventilation for babies with chronic lung disease.
Back