High Frequency Oscillatory Ventilation
DEFINITION:
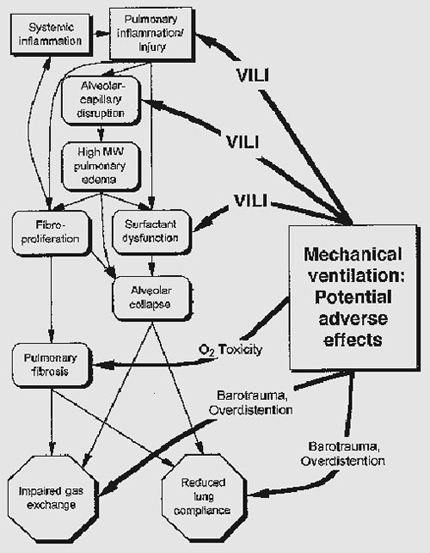
It is a mode of ventilation that executes supra-physiological breath rates and tidal volumes frequently less than dead space. The use of high frequency ventilation at low tidal volume allows the primary goals of ventilation, oxygenation and CO2 removal, to be achieved without the costs of pressure-induced lung injury.
Introduction
This innovative form of ventilation first described by Lunkenheimer in 1970's. During conventional ventilation direct alveolar ventilation accomplishes pulmonary gas exchanges and is based on the principle that the amount of gas reaching the alveoli equals the applied tidal volume minus the dead space volume. At the tidal volume below that of their anatomical dead space, sufficient gas exchange fails to occur.
Deleterious consequences of volume and pressure changes at alveolar level due to conventional mode of ventilation, which account for the significant variations in lung morbidities such as:
1. PIE
2. Pneumothorax,
3. Chronic lung disease
Potential Advantages of HFOV are
1. Uniform inflation of the lung fields
2. Improves gas exchange
3. Improves lung mechanics
4. Enables stable lung inflation
o Allows recruitment of alveolar space
o Reduces the risk of volutrauma
o Reduces risk of high peak airway pressure (PIP)
o Reduces the risk of airway stretching
o Improves V/Q matching
5. Reduces air leak
6. Decreases the amount of inflammatory mediators and alveolar edema
7. Prevents the development of hyaline membrane disease (HMD)
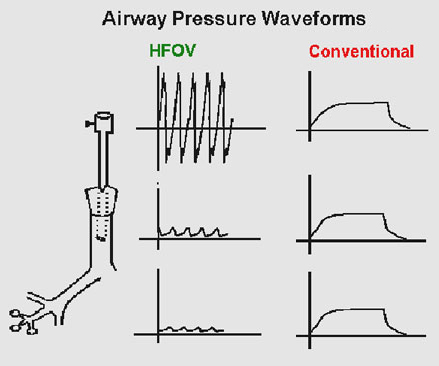
CONVENTIONAL VENTILATION vs. HFOV
This use of positive pressure ventilation has its side effects (Fort et al 1997). These are briefly described below;
1. Decreased cardiac output: The use of Positive End Expiratory Pressure (PEEP) will further decrease venous return and, thus, cardiac output.
2. Decreased urine out put: As the cardiac output fall, the kidneys attempt to retain fluid.
3. Risk of ventilator associated pneumonia.
4. Risk of tracheal and lung damage if gases are not humidified.
5.VILI due to high or increasing airway pressures.
It is the potential risk of barotrauma which HFOV attempts to deal with, and which will now be dealt with in more detail. Ventilating patients with either decreased lung compliance and/or increased lung resistance can lead to alveolar and lung damage and exacerbate their respiratory problems (Simma et al 2000, Weavind and Wenker 2000). HFOV is generally considered to be of benefit for patients with diseased lungs for a number of reasons;
1. It uses smaller tidal volumes than conventional ventilation. To try to deliver a constant tidal volume to a patient with increasingly 'stiff' lungs results in further lung complications.
2. HFOV keeps the lungs/alveoli open at a constant (Open lung type), less variable, airway pressure. This prevents the lung 'inflate-deflate', inflate-deflate' cycle, which has been shown to damage alveoli and further complicate lung disease (Fort et al 1997).
3. Along with the above lung protection strategy, it is believed that HFOV enhances gas mixing and improve ventilation/perfusion (V/Q) matching (Fort et al 1997).
Thus, patients who are at risk of further lung damage due to increases in airway pressure secondary to increases in resistance and decreases in compliance, HFOV can be considered an alternative.
INDICATIONS:
Rescue: HFOV indicated on a case-by-case basis and only in consultation with experienced senior personnel.
Prophylactic: HFOV even though shows a significant reduction in chronic lung disease,5 the findings are not consistent across the trials and doubts remain about the high incidence of brain injury which was found in the HiFi trial, the first and largest of these kind.
PRINCIPLES
HIGH FREQUENCY Oscillation involves complex mechanisms of gas exchange, which are not fully understood. In most situations gas exchange occurs with tidal volumes less than the anatomical dead space. In HFV considerable mixing of fresh and exchanged gas in the airways and lungs is believed to be the key to the success of this innovative ventilatory technique.
HFOV has been described as “ wobbling CPAP "
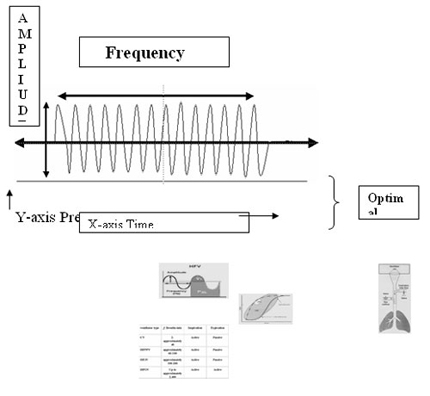
CPAP=MAP which provides Sustained inflation and recruitment of lung volume to achieve oxygenation. Wobbles depends on two parameters such as Frequency set (Hz) and oscillating pressure waveform or amplitude (?P) on the set MAP, in-turn helps in Alveolar ventilation and CO2 removal.
Evidences shown that
It allows for pulmonary gas exchange at lower mean airway pressures than conventional mechanical ventilation.
The current literature does support the preferential use of HFV over CMV in conjunction with iNO to maximize oxygenation in hypoxemic respiratory failure, in particular, as a result of persistent pulmonary hypertension.
HFV has become a reliable and useful addition to the various modes of mechanical ventilation in neonates.
There is no evidence, that elective use of HFV, in the form of HFOV or HFFI, provides any greater benefit to premature infants who have RDS than CMV [35].
 Postulated methods of Gas-Exchange in HFOV:
Postulated methods of Gas-Exchange in HFOV:
HFOV uses a range of different mechanisms, including facilitated diffusion, rather than conventional bulk flow of gases to achieve adequate ventilation and that tidal volume (VT) in HFOV is less than deadspace (VD) (8, 9). Normal physiologic volumes for infants are a VT of 6–9 mL/kg and a VD of 2–2.5 mL/kg (12). Recent attempts at lung-protective strategies during CMV have aimed for VT ranges of 4–6 mL/kg. Minute ventilation during CMV is defined as VT X rate, whereas several studies have shown that VT is much more influential in CO2 elimination during HFOV given that minute ventilation is defined as ([VT/kg]2 X f), ([mL/kg]2 X Hz),high-frequency minute ventilation (HFMV)
1 Taylor dispersion (axial and radial augmented dispersion)

a. Conventional and b. HFOV ventilation
Can produce a mixing of fresh and residual gas along the front of a flow of gas through a tube
2 Augmented diffusion.
Can occur at the alveolar level secondary to the added kinetic energy from the oscillations.
3. Bulk axial flow
Bulk flow can provide conventional gas delivery to proximal alveoli with low
regional dead space volumes.
4. Intra-alveolar pendelluft
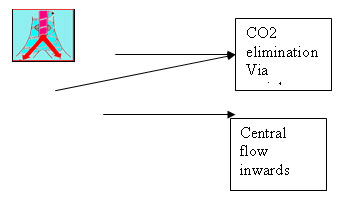
Pendelluft can mix gases between lung regions having different impedances.
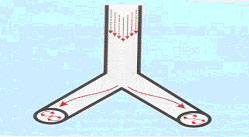
5. Coaxial flow
Gas in the center flows inward, while gas on the periphery flows outward. This develops because of the asymmetric low profile of high velocity gases.

It has been suggested by MacIntyre (1998) that perhaps all of the above may be operative simultaneously during HFOV
6. Augmented molecular diffusion can occur at the alveolar level
secondary to the added kinetic energy from the oscillations
Principles of operation
- This utilizes a piston –diaphragm to produce oscillatory gas flows within the airway.
- A vibrator diaphragm moves a small volume of gas toward and away from the patient.
- A continuous gas flow eliminates C02 build up and delivers 02. It allows continuous gas flow to escape while maintaining vibration of gas in the airway.
- Bias flow is delivered to the proximal airway in order to provide a supply of oxygen and a means of CO2 removal.
- Lung volume is maintained above FRC by the use of a constant distending pressure determined by end-expiratory or mean airway pressure.
- Frequencies range from 3-20 Hz.
- Some oscillators have adjustable I:E ratios (Sensor medics); others are fixed (Dragger/Humming V).
- The ventilator monitors proximal airway pressure, not alveolar pressure.
A true high frequency oscillator moves a column of gas rapidly back and forth in the breathing circuit with a piston pump or a loudspeaker membrane; hence both inspiration and expiration are active. Flow interrupters are a hybrid variety of oscillators. (57)
True Oscillators
Flow Interrupters
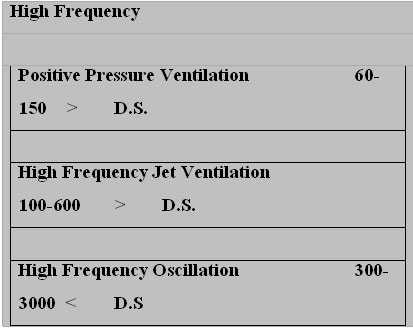
CONTROL VARIABLES IN HFO
The control of oscillatory ventilation rests on
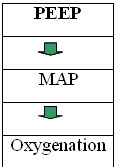
1) Mean airway pressure which directly controls oxygenation and around which the pressure oscillates
2) Oscillatory volume which results from the pressure swings and essentially determines the effectives of this form of ventilation
3) Oscillatory frequency (1hz= 60 breaths per minute), which determines the number of cycles per unit time.
Control Parameters
HFV: Control Parameter: 1
HFV: Control parameter: 2
HFV: Control Parameter: 3
HFOV : Clinical Indications :
When conventional ventilation fails
• Reduced compliance
• RDS/ARDS
• Air-leak
• Meconium aspiration
• BPD
• Pneumonia
• Atelectasis
• Lung hypoplasia
• PPHN
HFOV : Indications and guidelines (58) (59)
When conventional ventilation fails
Preterm Babies
Relative: PIP > 22 m bar
Absolute: PIP > 25 m bar
Term Newborns
Relative: PIP > 25 m bar
Absolute: PIP > 28 m bar
MAP > 14
How to initiate HFOV
Starting of HFV
Keep MAP (PEEP): 2-5 mbar (mmHg) above MAP of conventional ventilation, according to necessity (High Volume strategy) Increase MAP gradually until pO2 increases or
Good saturations above 98 % seen
After 30 min: X-Ray should show an expansion of 8-9-rib level HFV frequency: 10 Hz in term and 12-15 Hz in preterm babies HFV amplitude: 100% to start with
Watch Thorax
Vibrations
HFV: CLINICAL MANAGEMENT
Hypoxia: increase MAP upto maximum 25-mbar (if CVP does not increase) Alternatively: apply sustained inflation
Hyperoxia: reduce FiO2 down to about 0.6 - 0.4 very carefully. Then decrease MAP cautiously every 6-8hourly.
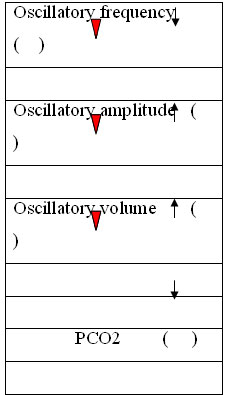
Hypercapnia:
Increase DCO2 amplitude 100%
Decrease HF –frequency (Normal range; 8-12 Hz)
Hypocapnia:
Decrease DCO2
Decrease amplitude
Increase frequency
Overinflation:
Reduce MAP cautiously
Decrease frequency
Hypotension/increase in CVP:
Volume expansion in hypotension Inotropes Dopamine/Dobutamine Reduce MAP
Control of O2
*MAP-Adjustment is done gradually by decreasing conventional rate (by 5 bpm at a time until conventional ventilatory rate is 0 breaths per minute) while increasing PEEP (by 1 cm H20 until we reached the MAP value set in the conventional ventilation). *It is very important to keep MAP constant during the conversion to HFO to prevent excessive atelectasis and loss of oxygenation. *The goal being a MAP equal to or slightly (1-3cm) above the previous MAP. In our center we opt to keep MAP 1-2 cm above MAP observed in conventional ventilation. (High Volume Strategy)
Control of CO2
Management according to ABG (Ventilation-Ve)
a. PaCO2 35-39 or 60-64 adjust AMP by=3 units to change PaCO2 + 1- 4 mm Hg
b. PaCO2<35 or >65 adjust AMP by=6 units to change PaCO2 + 5- 9mm Hg
c. PaCO2<30 or >70 adjust AMP by =9 units to change PaCO2 + >10 mm Hg
AMP: AMPLITUTDE or DELTA P
HFV: Weaning
1. Reduce FiO2 to 0.3 -0.5 as the lung improves.
2. Reduce MAP by 1 cmH2O (mbar) every 4-8 hourly if the FiO2 is less than 0.7.
Minimum HFO settings are MAP of 7 - 8cm H2O and Fi O2 of 0.4 chest expansion should not exceed 8-9 spaces on x ray
3. Reduce amplitude by 3units till the CO2 rises to acceptable levels. (50-55) A minimal amplitude is about 12-14 units or a delta P of around 30-35. Always observe for chest wall vibrations.
4. It is better to convert to the conventional mode of ventilation, *preferably in a volume mode before to make sure of spontaneous vigor in maintaining the tidal volume or Continue ventilation with IMV/SIMV with same MAP obtained from HFO at least for 24hrs before trying extubation.
5. Extubation from HFV is also possible if respiratory activity is sufficient.
EXTUBATION
Patients are ready to be extubated for a trial of NPCPAP when they meet the following criteria:
a. PEEP/MAP < 9-10 cm
b. FiO2<0.40 use NPCPAP of 7-9 cm
*In our center once the PIP/PEEP between 12-15/3-4 the and MAP is 6- 8 we extubate to NPCPAP of 5-6 cm of H2O.
COMPLICATIONS IN HIGH FREQUENCY VENTILATION (62)(63)
o Converting conventional ventilation to high frequency - inadequate experience
o Secretions in endotracheal tubes
o Water condensation in ventilator circuit
o Endotracheal suction-atelectasis
o Humidifier water level not optimal -loss of MAP
o Excessive mean airway pressure - hypotension
o Excessive amplitude of vibrations of chest wall - hypocarbia
CAUTION: High frequency ventilation should not be attempted in centers with inadequate experience with conventional ventilation. Reasonable expertise, continuous monitoring and working knowledge of it’s principles and application are essential.
PATHOPHYSIOLOGY
In order to understand the benefits of artificial ventilation, it is first important to understand respiratory failure.
Patients need to be intubated and ventilated in order to treat and manage respiratory failure (Oh 1997), of which there are two types
• Type1: hypoxaemia without CO2 retention. These will include asthma, pneumonia, pulmonary oedema and pulmonary embolism.
• Type2: hypoxaemia with CO2 retention. These will include chronic bronchitis,
post operative hypoxaemia, chest injuries and chronic lung disease.
Along with patients suffering from respiratory failure, there are certain patients who need ventilatory support for other medical reasons. Post operative ICU admissions for 'waking, warming and weaning' are not uncommon (Adam and Osborne 1997) and certain maxillofacial surgical patients require a period of post operative care/management on ICU, during which time the patient is kept sedated and ventilated.
CONVENTIONAL VENTILATION vs HFOV
Once a patient has been identified as needing artificial ventilation, they are intubated and placed on a ventilator and ventilated using positive pressure. Gases are delivered to the patient using pressure to inflate the lungs, expand the alveoli and allow for gas exchange and oxygenation (Weavind and Wenker 2000). Such delivery can be by means of pressure cycled, volume cycled and/or time cycled. However, the point to remember here is that whatever the mode of conventional ventilation used, they will all use positive pressure to deliver gas and achieve their ventilatory goals.
This use of positive pressure ventilation has its side effects (Fort et al 1997). These are briefly described below;
• Decreased cardiac output: Inspiratory pressure are higher than normal and will reduce venous return. Further, the use of Positive End Expiratory Pressure (PEEP) will further decrease venous return and, thus, cardiac output.
• Decreased urine out put: As the cardiac output fall, the kidneys attempt to retain fluid.
• Risk of ventilator associated pneumonia.
Risk of tracheal and lung damage if gases are not humidified.
Lung trauma due to high or increasing airway pressures.
It is the potential risk of barotrauma which HFOV attempts to deal with, and which will now be dealt with in more detail. Patients who develop Acute Respiratory Distress Syndrome (ARDS) will have reduced lung compliance and increases in their lung resistance (Simma et al 2000). Ventilating patients with either decreased lung compliance and/or increased lung resistance can lead to alveolar and lung damage and exacerbate their respiratory problems (Simma et al 2000, Weavind and Wenker 2000). HFOV is generally considered to be of benefit for patients with diseased lungs for a number of reasons;
1. It uses SMALLER tidal volumes than conventional ventilation. To try to deliver a constant tidal volume to a patient with increasingly 'stiff' lungs results in further lung complications. HFOV reduces this risk by delivering small tidal volumes.
2. HFOV keeps the lungs/alveoli open at a constant, less variable, airway pressure. This prevents the lung 'inflate-deflate', inflate-deflate' cycle, which has been shown to damage alveoli and further complicate lung disease (Fort et al 1997).
3. Along with the above lung protection strategy, it is believed that HFOV may enhance gas mixing and improve ventilation/perfusion (V/Q) matching (Fort et al 1997).
Thus, patients who are at risk of further lung damage due to increases in airway pressure secondary to increases in resistance and decreases in compliance, may benefit from HFOV. When conventional ventilation fails to safely and adequately provide respiratory support, HFOV can be considered an alternative.
HIGH FREQUENCY OSCILLATORY VENTILATION
Essentially, HFOV provides small tidal volumes (not really a tidal volume, but an Amplitude, usually referred to as Delta P: P) usually equal to, or less than, the dead space; 150 millilitres, at a very fast rate (Hertz-Hz) of between 4-5 breaths per second. The delivery of tidal volumes of dead space or less at very high frequencies enables the maintenance of a minute volume. Lungs are kept open to a constant airway pressure via a mean pressure adjust system. Further, HFOV allows for the decoupling of oxygenation from ventilation: it allows the clinician to separately adjust either oxygenation or ventilation.
This is a very simplified way of describing HFOV, and needs more detail if the principles are to be understood.
The core of a HFOV system will be a piston assembly. Cairo and Pilbeam (2000) describe the working of such a piston assembly very well;
"Such a system will incorporate an electronic control circuit, or square-wave driver, which powers a linear drive motor. This motor consists of an electrical coil within a magnet, similar to a permanent magnet speaker. When a positive polarity is applied to the square-wave driver, the coil is driven forward. The coil is attached to a rubber bellows, or diaphragm, to create a piston. When the coil moves forward, the piston moves toward the patient airway, creating the inspiratory phase. When the polarity becomes negative, the electrical coil and the attached piston are driven away from the patient, creating an active expiration."
The amount of polarity voltage applied to the electrical coil determines the distance that the piston is driven toward/away from the patient' s airway. Therefore increasing the polarity voltage increases the piston movement, or amplitude. The easiest way to conceptualise this polarity voltage, or amplitude, is to view it as the means by which tidal volumes are delivered, the greater the piston displacement (amplitude) the more volume delivered to the patient. It is the piston displacement which causes the oscillations. The extent to which the amplitude increases depends on the resistence the piston encounters to forward movement (Cairo and Pilbeam 2000). For example, when the oscillator is used with a patient with low compliance or high resistance, the piston meets greater pressure during the inspiratory phase.
Since tidal volumes are so low, gas transport mechanisms other than conventional bulk flow must be invoked to explain gas and CO2 flow. This will be explained a later.
Along with the above mentioned amplitude which provides ventilatory volumes, a Mean Pressure Adjust control knob allows for adjustments in mean airway pressure (Paw). This control varies the resistance placed on a mushroom shaped control valve on the patient circuit at the terminus of the expiratory limb. This allows the clinician to manipulate the Paw. Adjusting the Paw enables lung recruitment, keeps lungs and alveoli open at a consent pressure, thus avoiding lung expansion/collapse, lung expansion/collapse which is detrimental to the lungs. Research has also shown that increasing the Paw during HFOV does not effect cardiac out put, unlike conventional ventilation, and increases oxygenation (Fort et al 1997). The mean pressure adjust control is Bias Flow dependent. Bias flow is the rate at which the flow of gas, through the oscillator, is delivered to the patient.
The speed at which the oscillator runs is set by manipulating the frequency. The frequency control sets the breaths per minute in Hertz (Hz). One Hz is equal to one breath per second, i.e., 60 breaths per minute. A frequency of 5 Hz gives a frequency of 5 breaths per second, or 300 breaths per minute. An important point to remember is that as frequency is increased, the excursion of the piston is limited by the time allocated for each breath cycle. Thus, changes in frequency will effect Paw and the amplitude.
In conjunction with amplitude, mean airway adjust, bias flow, and frequency control, an oscillator will usually also allow for the inspiratory time to be adjusted. The inspiratory time will be displayed as % Inspiratory Time. Further, as with conventional ventilators, alarm limits can also be set.
USES FOR HIGH FREQUENCY OSCILLATORY VENTILATION
The use of HFOV in neonates and paediatric patients is well researched and established (Goldsmith and Karotkin 1998). However, its use with adults has only relatively recently been realised. Research is now being conducted into its use with adult patients.
The conceptual advantages of using HFOV are: smaller tidal volumes, a constant, less variable, airway pressure and the fact that nonbulk-flow mechanisms may improve V/Q matching. HFOV is used to avoid conventionally ventilating atelectasis prone lungs in ARDS (Clark et al 1994). Over distention of the lungs and ongoing atelectasis contribute to progressive lung injury which arises not directly from the disease process itself, but from the impact of the ventilator patterns used to support gas exchange during the course of the illness by conventional ventilation (Isabey et al 1984). Atelectasis can be halted, and even reversed, during HFOV, while avoiding the over distention so commonly seen with conventional ventilation (Froese 1997, Tseng et al 1998, MacIntyre and Branson 2001).
Thus, HFOV is used to minimize ventilator-related lung injuries in ARDS. The protective strategy of a constant airway pressure, with smaller tidal pressure swings, preventing over distention, are reasons why HFOV is used.
In addition to this better alveoli recruitment strategy, the rapid flow pattern may enhance gas mixing and improve V/Q matching. However, since tidal volumes are smaller than usual, gas transport mechanisms other than conventional bulk flow transport must be discussed to explain oxygen and CO2 flow. There are a number of mechanisms to explain gas transport under these non-physiologic conditions. The following have been suggested by Weavind and Wenker (2000):
• Bulk flow can still provide conventional gas delivery to proximal alveoli with low regional dead space volumes.
• Coaxial flow. Gas in the centre flows inward, while gas on the periphery flows outward. This can develop because of the asymmetric low profile of high velocity gases.
• Taylor dispersion can produce a mixing of fresh and residual gas along the front of a flow of gas through a tube.
• Pendelluft can mix gases between lung regions having different impedances.
• Augmented molecular diffusion can occur at the alveolar level secondary to the added kinetic energy from the oscillations The importance of each of these is debated. It has been suggested by MacIntyre (1998) that perhaps all of the above may be operative simultaneously during HFOV.
The combination of these non-physiological, non bulk flow gas mechanisms and a constant airway pressure, are the advantages of HFOV over conventional ventilation. Improvements in V/Q matching and the preventing of over distention have led HFOV to be viewed as an alterative to conventional positive pressure ventilation. In a study by Fort et al (1997) HFOV was evaluated in terms of safety and effectiveness in patients with ARDS and with whom conventional ventilation had failed. This prospective study (n=17) included patients who had failed conventional ventilation, had very high peak inspiratory pressure (peak pressure of 54.3 +/- 12.7cm H2O), a PaO2/FiO2 ratio of 68.6 +/- 21.6 and positive end expiratory pressure of 18.2 +/- 6.9cm H2O. HFOV was instituted after varying periods of conventional ventilation (5.12 +/- 4.3 days). A lung volume recruitment strategy was employed, consisting of incremental increases in mean airway pressures to achieve a PaO2 of > or to 8.0 kPa. During the study 13 patients demonstrated improved gas exchange and an overall improvement in PaO2/FiO2 ratio. Cardiac output was not compromised in any of the patients, despite increases in mean airway pressure. The authors of the study maintain that HFOV is both safe and effective in adult patients with severe ARDS failing conventional ventilation. They do, however, acknowledge the need for continual research into HFOV in adult patients who fail conventional ventilation.
COMPLICATION OF HIGH FREQUENCY OSCILLATORY VENTILATION
A number of complications of HFOV have been identified in the literature.
Although approved for use and despite the research into the effects of HFOV, oscillatory ventilators are still, largely, experimental devises (Goldsmith and Karotkin 1998). There are a number of devises available and this raises the issue of staff training. Generalisation for one oscillator may not be applicable to another (Goldsmith and Karotkin 1998).
The possibility of lung over distention due to trapping of gas has also been investigated(Boros et al 1985). Such distending pressure is commonly called inadvertent PEEP. Since this can not be measured directly, the exact extent to which this is a problem is controversial. As is the problem of lung under distention. In normal circumstances, small tidal volumes delivered at a constant mean airway pressure may actually exacerbate, and indeed result in, progressive atelectasis, one of the problems HFOV is thought to overcome!! Again, this is controversial (Goldsmith and Karotkin 1998).
A number of studies have linked high frequency ventilation to tracheal inflammation and a condition called Necrotizing Tracheobronchitis (NTB) (Boros et al 1985, Wilson et al 1987, Goldsmith and Karotkin 1998). These conditions highlighted the vital need of adequately humidifying respiratory gases. To summarise the above, HFOV :
• Enables stable lung inflation
• Allows recruitment of alveolar space
• Reduces the risk of volutrauma
• Reduces risk of high peak airway pressure
• Reduces the risk of airway stretching
• Improves V/Q matching
NURSING A PATIENT RECEIVING HIGH FREQUENCY OSCILLATORY VENTILATION
When nursing a patient on an oscillator, there are a number of specific nursing aspects that should be highlighted. The sight of someone being 'oscillated' can be disturbing for the family and friends of the patient (ManIntyre and Branson 2001). It is therefore essential to ensure adequate information is provided by the nurse to the patient' s family and friends. After a patient has been attached to an oscillator, the Paw will be increased. Observation of the patient for equal and continuous chest vibrations should be performed. This is known as the 'chest wiggle factor' . Chest wiggle is more accurate than using terms such as 'belly wobble', as not all patients have wobbly bellies!
Chest wiggle must be evaluated upon initiation and followed closely thereafter. If chest wiggle diminishes it may be that the ET tube has moved or is obstructed. Chest wiggle on one side only may indicate that the patient has developed a pnueumothorax. Chest wiggle assessments should be thereafter performed following any patient re-positioning.
It is nigh on impossible to auscultate the chest in the normal way whilst a patient is on an oscillator. Because the movement of gases through the lungs is different during HFOV, nurses must rely on other clinical signs. Listening to the piston via the chest has been suggested. The clinician can listen to the intensity or sound that the piston makes throughout the chest. However, what sounds the clinician is supposed to hear is debated and unclear. It is generally deemed, therefore, unnecessary to perform chest auscultation during HFOV.
A closed system suction unit should be used. It is not necessary to disconnect the patient to suction as this will potentially de-recruit lung volumes. Unless otherwise indicated, suctioning for the first 24 hours is not necessary. When using a closed system suction system, it is important to draw back the suction catheter all the way from the ET tube on completion. Ideally, the patient would be thoroughly suctioned before HFOV is commenced (Senormedics 1998). The point at which the ET tube is cut and secured at the lips should initially be noted. This measurement will act as a reference point in case there is confusion over whether the ET tube has moved.
ET tube position should be checked regularly. When the suction system is changed, two nurses will be needed to ensure safety. Once the patient is oscillated, the nurse must try not to disconnect the patient from the oscillator, or de-recruitment may occur. This is a controversial point. There is very little research/evidence into de-recruitment following disconnection from an oscillator. Further research is needed in order to establish the problem of disconnection associated de-recruitment. The nurse must monitor blood gases, specifically PO2 and PCO2 and monitor cardiovascular status continuously (Kidd 1988). The nurse must also set alarm limits to within safe and acceptable boundaries.
The recognising of possible complications will involve the nurse being able to recognise ET tube obstruction (amplitude will increase, SpO2 will decrease and CO2 will increase). Recognise pneumothorax (decrease in SpO2, dissimilarity in the height of the left and right chest walls and a fall in blood pressure). To be able to recognise possible lung over-distension (Fall in blood pressure, increase in central venous pressure and decrease in SpO2). Should the nurse suspect any of these complications, then informing the appropriately trained medical staff is of, obvious, importance.
When positioning a patient, it is recommended that at least two nurses assist with ET tube protection to ensure that patient- ET tube disconnection does not occur (Sensormedics 1998). .
Finally, it is very important to humidify gases before they are delivered to the patient. A standard Fisher and Paykel system, for example, adequately humidifies gas and helps to prevent Necrotizing Tracheobronchitis (Goldsmith and Karotkin 1998).
SUMMARY OF NURSING CARE AND DUTIES
• Perform thorough suction before connecting to the oscillator.
• Assess patient upon commencement of HFOV. Monitor vital signs.
• Check for changes in pitch/rhythm of delivered breaths. Check chest wiggle and changes in chest wiggle.
• Use closed system suction catheter. Check position of ET tube. Never change closed system catheters on your own.
• Always humidify gases.
• If oscillator stops during suctioning; silence alarm, pull back catheter and restart oscillator.
• Observe for signs of pneumothorax, ET tube blockage and/or lung over-distension.
• Obtain blood gases and chest x-ray within first hour of commencement.
• Avoid disconnection.
• Ensure appropriate relative information regarding the oscillator.
CONCLUSION
In conclusion, then, HFOV improves V/Q matching, enables decoupled oxygenation and ventilation improvements, at a constant airway pressure. This means that pressure swings are reduced and high peak airway pressure are avoided.. Atelectasis is minimalised. Although HFOV is not without its complications, research seems to suggest that it is an alternative for the patient with ARDS who has failed conventional ventilation.
Specific nursing aspects need to be observed when nursing an oscillated patient.
Indications for using HFOV are unclear but do include:
??Air leaks such as pneumothorax and pulmonary interstitial emphysema
??To reduce barotrauma when conventional ventilator settings are getting very high
??When conventional ventilation is failing, particularly in meconium aspiration syndrome, pneumonia, persistent pulmonary hypertension of the newborn, and pulmonary hemorrhage.
It is important to initiate high frequency ventilation early if conventional methods of ventilation are failing. Normal blood gas values are listed below and are usually recorded in the following order:
pH:
PaCO2:
PaO2:
HCO3:
BE:
7.35-7.45
35-45
80-100
22-26
+/- 5
Impending respiratory failure is indicated by rising carbon dioxide levels and dropping oxygen levels despite ventilator adjustments to compensate. Respiratory failure is defined as a PaCO2 above 55 and a PaO2 below 50.
The lung parenchyma in children is especially delicate and the high peak pressures utilized in conventional methods of ventilation can be harmful to the delicate lung tissue. High frequency ventilation offers the advantage of improved CO2 removal at lower peak pressures. Barotrauma occurs when the lung tissue is damaged due to the excessive pressures that can be created by conventional ventilation. However, high frequency ventilation can cause hemodynamic changes. This is due to the continuous pressures that are generated by high frequency ventilation, which cause increased pressures in the thoracic cavity.
Why is this important?
Increased thoracic pressures impede cardiac output. This, in turn, affects the blood pressure, and a vicious spiral can be created. In order to avoid this, it is important to carefully monitor the patient’s hemodynamic status.
Continuous pressure monitoring is crucial. Arterial lines are necessary when a pediatric patient is placed on high frequency ventilation. In addition to the continuous blood pressure monitoring, the arterial line also functions as the site of numerous blood draws for arterial blood gases. The ideal end results when using high frequency ventilation are no adverse damage to the lungs due to ventilation, and no neurological deficit. Prolonged periods of inadequate oxygenation can cause permanent brain damage.
It is important to know when to implement HFOV. High frequency ventilation does not have to be the last resort prior to extraordinary measures such as extra corporeal membrane oxygenation (ECMO). ECMO is a system similar to cardiac bypass, where the blood is oxygenated and CO2 is removed while the blood is outside of the body. Each facility using high frequency ventilation must have a clear definition of when it is to be implemented.
The goal of positive pressure ventilation, in any form, is for a complete recovery of lung function without any adverse effects. When preparing to change from conventional to high frequency ventilation, what preparations must be made? ??It is advantageous to have arterial line catheterization established. If the patient is a neonate, this may be an umbilical artery catheter. In pediatric patients, this can be any catheter placed in an artery. In addition, continuous monitoring of vital signs is important and includes:
• ?Air leaks such as pneumothorax and pulmonary interstitial emphysema
• To reduce barotrauma when conventional ventilator settings are getting very high
• When conventional ventilation is failing, particularly in meconium aspiration syndrome, pneumonia, persistent pulmonary hypertension of the newborn, and pulmonary hemorrhage
pH:
PaCO2:
PaO2:
HCO3:
BE:
7.35-7.45
35-45
80-100
22-26
+/- 5
??Blood pressure monitoring is especially important because high frequency ventilation can cause hemodynamic changes such as hypotension and/or decreased cardiac output. If the use of high pressure leads to a decrease in cardiac output, tissue and organ perfusion are affected. By carefully monitoring the urine output, kidney function, and thus organ perfusion, can be monitored.
??As thoracic pressures change with the administration of high frequency ventilation, negative effects may result. It is especially important to obtain a chest x-ray (CXR) 30 to 60 minutes after the initiation of high frequency ventilation. This helps to determine whether or not the mean airway pressures are compressing the heart.
??Expansion of the chest also must be monitored. The expansion of 8.5 to 9 ribs indicates proper inflation. Adjustments should be made to correct the expansion, as needed. As the lungs heal, they become more inflated; hyperinflation may lead to pneumothorax. Regular chest films must be taken to determine improvement or decompensation. Initially, chest x-rays should be taken every six hours. The care of patients receiving high frequency ventilation is very individualized. Policies regarding CXR intervals are set by each facility.
Nitric Oxide is frequently used in conjunction with HFOV. It is a potent pulmonary vasodilator and works specifically on the pulmonary bed to help improve oxygenation.
ettings
Amplitude High Frequency Ventilation is much more dependent on amplitude than on rate. How are the initial settings for high frequency ventilation determined? The first setting to consider is the amplitude, or delta P. The amplitude is similar to the tidal volume in conventional ventilation. The proper setting is determined by observing the patient to see how much chest
excursion/movement occurs with oscillation. This setting should be enough to vibrate or "wiggle" the thorax from the nipple line to the umbilicus. Settings are changed by 1-2cm H2O until the desired amplitude is reached. A good initial amplitude setting is 2 cm H2O. There is no exact calculation for setting the amplitude.
Changes in the amplitude require readjustment of the mean airway pressure.
Frequency The frequency, which is similar to the rate, is measured in hertz. There are rules by which to choose the setting for the hertz. Multiply the number of hertz times 60 and the exact rate is calculated. Below is a chart for determining initial hertz settings.
1000 grams 15 Hz
1000-2000 grams 12 Hz
2.0-10 Kg 10 Hz
13-20 Kg 8 Hz
21-30 Kg 7 Hz
> 30 Kg 6 Hz
For Meconium Aspiration Syndrome 3-6 Hz
This table is taken from the recommendations by Sensormedics for use with the 3100A high frequency oscillating ventilator. Settings depend on the size of the patient and the disease process. Changes in frequency dramatically
??Blood pressure ??Cardiac rate and rhythm ??Respiration ??Oxygen saturation
1000 grams 15 Hz
1000-2000 grams 12 Hz
2.0-10 Kg 10 Hz
13-20 Kg 8 Hz
21-30 Kg 7 Hz
> 30 Kg 6 Hz
For Meconium Aspiration Syndrome 3-6 Hz change the amplitude and MAP.
When initiating high frequency ventilation in the pediatric patient, begin with a hertz such as 6 or 7. This will allow for the greater lung capacity and lung volumes of older patients. Once again, the amplitude should be in accordance with the amount of "wiggle" seen on the chest.
When ventilating the pediatric patient, adequate cardiac output must be maintained. The blood pressure must be within acceptable parameters. Without adequate circulation, there cannot be adequate tissue oxygenation.
What steps are to be taken if the blood pressure is not adequate?
??The first step is a fluid bolus of normal saline.
??Consider increasing the rate of intravenous maintenance fluids. This should improve blood pressure and cardiac output. If this is not sufficient, vasoactive infusions such as dopamine should be considered. These can be instituted in addition to the increase in intravenous fluids.
??Input and output must be continually monitored in order to prevent fluid overload.
Mean Airway Pressure
The mean airway pressure (MAP) is to HFOV as peak airway pressure is to conventional ventilation. For diffuse alveolar disease, the initial mean airway pressure should be set approximately 2-4 cm H2O over what the value for peak pressure was on the conventional ventilator. If compression of the heart is noted on CXR, a decrease in the mean airway pressure is necessary. The recommended decrease is .5 to 1 cm H2O on the MAP (mean airway
pressure) dial. A spontaneous pneumothorax is a complication that can result from high frequency ventilation due to a high mean airway pressures. Optimal pressures are desired at all times with high frequency ventilation. Optimal pressures can be defined as the amount of pressure necessary to ventilate adequately and maintain a normal PaCO2.
FiO2
An initial FiO2 is selected to optimize pulmonary vascular resistance while the ventilator is opening the lungs. Use caution when administering high concentrations of oxygen to the neonate. Paralytic agents may cause fluid retention, and this should be taken into consideration. The patient may be paralyzed initially, but paralytics should not be used for an extended period of time. As soon as tolerated by the patient, the agent should be discontinued
Case Presentations
The following are case presentations of patients with whom high frequency ventilation was used. There is a discussion of how each case progressed and the reasoning behind each decision that was made. Each patient is different and may respond differently to the clinical decisions made. It is important to assess and reassess the patient after each change. Vital signs are normal unless otherwise stated.
Case 1
A 27 week gestational premature infant with an admission weight of 1095gm is admitted to rule out sepsis. The infant had been delivered to a G2P1 32-year-old mother who had received adequate prenatal care. He is admitted for pneumonia, fever 103'F, and a left upper lobe infiltrate. Upon delivery, the infant's pH is 6.90 and he is intubated with a 2.5 fr endotracheal (ET) tube. Positive pressure ventilation is administered with 100% O2, and he is given intravenous plasmanate. A repeat umbilical blood gas pH is 7.10. Five meq NaHCO3 are given intravenously and blood cultures are drawn. Intravenous Ampicillin and Gentamicin are given. Another umbilical blood gas is drawn with results of pH 7.38/CO2 37/PO2 111/HCO3 17/-8.7. An initial dose of Survanta is given (4.3 cc). This artificial surfactant is useful in immature lungs to help the alveoli stay open, which improves oxygenation. Upon arrival to the NICU, oxygen saturations are sustained in the 80's by bagging with 100% oxygen. The infant is then placed on the high frequency oscillating ventilator with 100% O2. Oxygen saturations fall into the 60's and the infant is then re-intubated with a 3.0 endotrachael tube. Nitric Oxide is started at 20 ppm for probable persistent pulmonary hypertension secondary to sepsis/pneumonia. Dopamine is started at 5mcg/kg/min to improve renal function and organ perfusion. APGAR scores are 2 (1 min), 4 (5 min), and 6 (10 min). These are significant because the normal APGAR is 9 or 10.
What would be appropriate initial oscillator settings? Why?
??Initial oscillator settings are: FiO2 100%, Mean airway pressure (MAP) 17, Amplitude (Amp) 26,Hertz (Hz) 15. The correct guidelines for selection of the initial amplitude settings are to deliver enough amplitude to see a "wiggle" from the nipple line to the umbilicus. The FiO2 should initially be set at 100%.
The ABG results on these settings are 7.38/41/45/24/-0.6.
What is the main problem with this blood gas and what intervention(s) need to be taken?
??This blood gas shows adequate acid/base balance, but severe hypoxia: the main problem is oxygenation.
??An increase in the MAP can be instituted to increase oxygenation. This will open the airways more, similar to the way positive end expiratory pressure (PEEP) does in conventional ventilation. The guideline for increasing the mean airway pressures is to increase in increments from 0.5 to 1.0 cm H2O. The MAP was increased to 18.
Nitric oxide, recently approved by the Food and Drug Administration for treating critically ill newborns, is also started at this time. Nitric oxide is a pulmonary vasodilator and is indicated for the treatment of neonates with hypoxic respiratory failure. It dilates the blood vessels of the lungs so they can carry more oxygen and is used in conjunction with ventilatory support and other appropriate agents.
The follow-up blood gas is 7.28/65/76/30/+1.6.
Interpret this gas and determine the appropriate intervention.
??This result reflects an uncompensated respiratory acidosis.
??In order to decrease the CO2, the amplitude is increased to 30.
A repeat blood gas is drawn after the change in HFOV settings. The results are 7.35/51/172/28/+1.7
Is this an acceptable blood gas? Do any interventions need to be made and, if so, what are they?
The amplitude is increased to 32 in order to decrease the CO2 to within normal limits (35 – 45). The oxygenation status is excellent, therefore the FiO2 is decreased to 90%. This is a premature neonate; prolonged exposure to high amounts of oxygen has the potential to cause many problems, such as blindness. The ABG results after the changes are made are 7.38/42/74/24/0.2. Later ABG results are 7.59/27/144/25/+6.0.
Interpret this gas and explain the interventions.
??This gas reflects a respiratory alkalosis with hyperoxygenation.
??The amplitude is decreased to 22 to allow the CO2 to rise, and the FiO2 is further decreased to 80%.
Suddenly, the oxygen saturation drops to the 70's. The infant is suctioned for a moderate amount of blood. When placed back on HFOV the saturation then falls into the 40's and the heart rate drops to the 80's. A large amount of frank blood is suctioned from the ET tube. Epinephrine is instilled into the endotracheal tube to assist in hemostasis.
A portable chest x-ray is taken.
What is causing the above condition?
??Due to the sudden onset of hemodynamic changes, a pneumothorax is suspected and the portable chest xray shows a large left pneumothorax.
A 10fr argyle chest tube is place in the fourth intercostal space, mid-axillary, with an audible resolution of the pneumothorax. Epinephrine is a vasoconstrictor and is used in this situation to slop bleeding in the lungs. Any time intrathoracic pressure increases, there is an increased risk for pneumothorax. As the mean airway pressures are increased, the risk for potential negative side effects are increased; the lung tissue in premature neonates is very delicate.
The ABG results after chest tube placement are 7.31/35/129/18/-8.0.
Interpret this gas and give the intervention.
??This is a partially compensated metabolic acidosis with hyperoxygenation.
??This acidosis has already been partially compensated for by blowing off CO2 and now must be treated pharmacologically. ??Sodium bicarbonate is given intravenously.
As the pneumothorax resolves, the infant is weaned off high frequency ventilation. As the neonate grows, so does the lung tissue. The infant is able to be changed to conventional ventilation and later extubated. He is then placed on oxygen via nasal cannula at an FiO2 to maintain the oxygen saturation greater than 92%. The infant is discharged thirty days after extubation. Case 2
A 14-year-old female is admitted with a decreased level of consciousness and abdominal bleeding. She also complains of abdominal pain, diaphoresis, and cramping in the left upper quadrant and periumbilical area. A diagnostic laparoscopy is performed, noting copious bleeding from a hemorrhagic cyst. She experiences hypotension and a decreased hemoglobin and hematocrit. Packed red blood cells are transfused. After fluid and blood resuscitation, an exploratory laparotomy is performed. A 2000cc hemoperitoneum with a small amount of
bleeding from the right ovary is discovered. The initial ABG on room air is 7.32/52/68/26.
What is happening? From the given data, is her condition prone to deteriorate? What are appropriate initial ventilator settings? ??This blood gas indicates a potential for respiratory failure. The CO2 level is higher than normal without any form of compensation. Due to this lack of compensation, we determine that this is an acute event.
This patient is intubated to protect her airway. Because of the potential for respiratory failure, it is preferable to intubate as an elective procedure, rather than as an emergency measure during an arrest situation. This patient has a great risk for hemorrhagic shock because of the amount of blood loss from the cyst. Close monitoring of the hematocrit and hemoglobin is important. In addition, it may be necessary to place an arterial catheter for continuous blood pressure monitoring and laboratory draws.
Despite all efforts using conventional ventilation via pressure regulated volume control (PRVC), a marked decrease in the PaO2 ensued. PRVC is a mode of ventilation with the Servo 300 that allows volume ventilation with minimal pressures. This minimizes barotrauma, but ventilates very effectively in difficult to ventilate patients. The ABG results are 7.47/31/54/22/+1.0 on settings of: rate- 20, tidal volume- 350, and FiO2- 100%.
Is this a ventilation or oxygenation problem?
??The pH is normal, the PaCO2 is only slightly below normal, and the PaO2 is 54; this is clearly an oxygenation problem. High frequency oscillation ventilation is initiated due to the need to minimize the insult to the lungs and try to recruit more alveoli and improve oxygenation.
What would be appropriate initial settings for HFOV?
??Initial ventilator settings are FiO2 100%, Hz 6.5, amp 45, and MAP 35.
??The amp setting is set just high enough to cause a wiggle from the nipple line to the umbilicus.
The amp setting is set just high enough to cause a wiggle from the nipple line to the umbilicus.
The initial ABG results are: 7.32/48/203/25/-1.5.
What should be done next?
Oxygenation has improved, but the PaCO2 is increasing while the pH is decreasing. This is indicative of respiratory acidosis. ??The goal here is to decrease the CO2. Hyper-oxygenation is not necessary.
What is necessary to achieve this goal?
??First, a decrease in the FiO2 is necessary to bring the PaO2 nearer to normal.
??Increasing the amplitude will help decrease the PaCO2.
Her condition seems to be stabilizing. The next ABG that is drawn shows the results: 7.04/113/376/30/-4.8.
Does this ABG indicate acidosis or alkalosis; metabolic or respiratory? What interventions should be taken? Should sodium bicarbonate be given? Why, or why not?
??The last blood gas indicates a severe respiratory acidosis. Because the bicarbonate level is 30, there is little metabolic involvement. In fact, the bicarbonate levels have started to rise to help the pH increase. In other words, there is some compensation. This is having no effect on the pH, which is still grossly abnormal.
??Sodium bicarbonate is not indicated for this blood gas because the cause is respiratory, not metabolic.
A chest x-ray is taken at this time and reveals rib expansion to the eighth rib. The mean airway pressures will expand the lungs to various rib expansions. The patient is suctioned for a large amount of thick secretions. The MAP is increased and the repeat ABG results are 7.13/93/225/31/+1.5.
What does this indicate? What are the options for the ventilator?
??Because the rib expansion should include 8.5 – 9 ribs, the MAP is increased to help improve oxygenation. ??In addition, the Hz may also be decreased to lower the CO2. The Hz is decreased to 5 and the amp is increased to 50. This will allow a faster rate and larger tidal volume.
The repeat ABG is 7.16/66/314/23/-7.0.
The MAP is then decreased to 27.7 and the Hz is decreased to 4. The repeat ABG is 7.34/50/128/27/+1.0 Do you agree with these changes? Why?
The MAP was decreased due to evidence of hyper-oxygenation. To lower the CO2 even more, the Hz was decreased.
As this case progresses over the next five days, the FiO2 and the MAP are weaned gradually.
When would be an appropriate time to switch to conventional ventilation? Why?
The goal is to wean the mean airway pressure lower. When the mean airway pressure is about 5 cm H2O over where it was on conventional ventilation, it is time to change ventilation modes. The amplitude setting is another indicator that it is time to switch to conventional ventilation. As the patient recovers, the amplitude is weaned. This indicates that the lungs are healing and recovering. It is then time to change the mode of ventilation to a conventional mode. When the amplitude is weaned to a low number, such as 18 or 20, it also indicates it is time to change to conventional ventilation. When placing the patient back on conventional ventilation, the settings might be temporarily higher to allow the patient to compensate for
Conclusion
It is important to use high frequency ventilation to avoid irreversible lung damage.
Self-Assessment Questions
Questions
Ventilation to high frequency, remember these tips:
1. Set the MAP at 2-4 cm H2O over the value used on conventional ventilation.
2. Set the amplitude to see a "wiggle" from the nipple line to umbilicus.
3. Set the hertz according to table listed.
4. Set the FiO2 at 100%.
5. Obtain a chest x-ray approximately 30-60minutes after the initiation of high frequency ventilation.
6. The rib expansion on x-ray should involve 8.5 – 9 ribs.
7. Obtain frequent arterial blood gasses to monitor progress.
8. Break the circuit only when absolutely necessary. Suction the endotracheal tube as necessary.
9. Wean the ventilator settings as tolerated.
References
Bryan, A.C., Cox, P.N. (Oct 30, 1999). History of high frequency ventilation. Schweiz Med Wochenschr.129(43):1613-6 Eichenwald, E.C., Stark, A.R. (Dec, 1999). High frequency ventilation: Current Status. Pediatric Review. 20(12):e127- 33.
Lai, M.K., Jeng, M.J. et al. (Dec 1999). High frequency oscillatory ventilation in premature infants. Chung Hua I Hsuen Tsa Chin (Taipei). 62(12):879-85.
Morris, K. (Jan, 2000). Acute hypoxaemic respiratory failure in children. Intensive Care Medicine. 26(1):109-16. Watkins, S.J., Peters, M.J., Tasker, R.C. (Jan-Feb, 2000). One hundred courses in high frequency ventilation: What have we learned? European Journal of Pediatrics. 159(1-2): 134.
1. High frequency oscillating ventilation (HFOV) differs from conventional ventilation in that it uses:
a. low rates and high tidal volumes. b. high rates and low tidal volumes.
c. low rates and high peak pressures. d. high tidal volumes and low peak pressures.
3. HFOV is especially useful in neonates because:
a. the lung parenchyma is very delicate. b. the immature lung is difficult to ventilate.
c. the lung tissue is highly elastic. d. the lungs are very small.
4. When utilizing high frequency ventilation, it is important to monitor:
a. respiratory rate and blood pressure. b. head circumference and weight.
c. blood pressure and heart rate. d. heart rate and weight.
5. Hemodynamic changes such as decreased cardiac output may occur with the use of HFOV due to the:
a. high tidal volumes used. b. increased imposed respiratory rate.
c. decreased carbon dioxide levels. d. increased intrathoracic pressure.
6. What is a guideline for setting the initial mean airway pressure?
a. Begin with double the setting used on the previous convention ventilator.
b. Start at 2-4 cm H2O over the value in use on conventional ventilation.
c. As low as tolerated to give a desired PaO2.
d. Begin at the maximum and wean down.
7. Adequate lung inflation is exhibited by the expansion of:
a. 8.5 to 9 ribs. b. 6 to 7.5 ribs.
c. 7 to 9.5 ribs. d. all the ribs.
8. The amplitude setting in HFOV is similar to the tidal volume setting in conventional ventilators.
a. True b. False
9. The initial setting for the amplitude should be enough to cause:
a. a wiggle from the clavicle to the umbilicus. b. vibration of the sternum.
c. a wiggle from the nipple line to the umbilicus. d. vibration of the abdomen.
10. Your patient is a 32 week gestation neonate on HFOV whose blood gas results are pH 7.27/CO2 67/PaO2 70/HCO3=32/+1.5. You know these results indicate________________ and an appropriate intervention would be to
a. metabolic alkalosis: decrease the amplitude.
b. metabolic acidosis: administer sodium bicarbonate intravenously.
c. respiratory alkalosis: decrease the mean airway pressure.
d. respiratory acidosis: increase the amplitude.
11. An increased risk of pneumothorax exists with HFOV use due to:
a. the decreased mean airway pressures. b. the increased intrathoracic pressure.
c. the high tidal volumes used. d. the high oxygen percentage administered.
12. The following statement is TRUE
a. Decreasing frequency results in a lowered PaCO2
b. Controls for oxygenation and ventilation are the same
c. Frequency is the primary control for CO2 elimination in HFOV
2. The following statement is true
a. Tidal volumes delivered by HFOV are typically less than the physiological dead space of the patient.
b. HFOV is a volume controlled, pressure limited ventilator.
c. HFOV requires the use of a special endotracheal tube.
3. The 3100B’s method of oxygenation is very effective because it
a. Stimulates the production of endogenous surfactant
b. Maintains an open alveolus by not allowing critical closure, eliminating the need for constant re-inflation.
c. Utilizes a diffusion process called Brownian movement
4. Of the following, which describes the mechanics of ventilation used by the 3100B?
a. Active inspiration with passive exhalation
b. Active inspiration and active exhalation
c. Passive inspiration with active exhalation
5. Ventilation and CO2 exchange using the 3100B is best described by
a. Ventilation is a function of large tidal volumes at low Paw
b. Ventilation is a function of I:E ratio
c. Ventilation is a function of frequency and Vt2
6. Pulmonary Injury Sequence may progress by which of the following processes
a. Normal respiratory cycles (tidal volume ventilation) in a surfactant impaired lung
b. HFOV with topical steroids and sympathomimetic Rx.
c. Over-stimulation of smooth muscle tissue caused by histamine release
7. HFOV in ARDS is most efficiently administered
a. After the patient has been on conventional mechanical ventilation for at least one week
b. With an initial Paw of 5cmH2O above conventional Paw
c. When the patient has marginal hemodynamic function
8. Choose the proper sequence for management of CO2 elimination
a. Frequency, amplitude, I:E ratio
b. I:E ratio, Frequency, Amplitude
c. Amplitude, Frequency, I:E ratio
9. The control of mean airway pressure in the 3100B is regulated by
a. Restricting the bias flow past the green balloon valve
b. Using an external PEEP valve
c. An electronic pressure transducer
10. Increasing the Power Control will most likely result in the following
a. A drop in minute ventilation and a rise in PaCO2
b. An increase in minute ventilation and a rise in PaCO2
b. An increase in minute ventilation and a drop in PaCO2
11. Which alarms stop the oscillator and opens the circuit pressure to atmospheric pressure?
a. Paw > 60 cmH2O or Paw < 5 cmH2O
b. Volume limit
c. High or Low Mean Airway Pressure Limit
12. Delta-P or Amplitude is primarily attenuated by the following
a. Endotracheal tube size
b. Patient weight
c. Patient diagnosis
v
13. A diminished chest wiggle along with a drop in SaO2 might signal the following
a. Improved compliance
b. Need for suctioning
d. Drop in cardiac output
14. The Patient Circuit Calibration procedure should be performed
a. At least every 500 hours
b. Whenever switching patient circuits or circuit components
c. Only when putting a new patient on the 3100B
15. Erratic Paw readings can be caused by
a. Low voltage to the oscillator magnet
b. A change in the air or oxygen line pressures
c. Spontaneous breathing
16. Adequate chest movement for an adult patient on HFOV can best be described as
a. Visible down to the patient’s toes
b. Above the diaphragm
c. From the chest to mid-thigh
17. A deliberately induced endotracheal tube cuff leak may achieve the following
a. Cause a rise in PaCO2 due to a drop in delivered volumes
b. Cause a drop in PaCO2 due to increased wash-out by the bias flow
c. Cause a rise in delivered Paw
18. Auscultation of heart and bowel sounds is best accomplished by
a. Stopping the oscillator for 20-30 seconds. Paw will be maintained.
b. Shutting off the ventilator
c. Auscultation should be performed with the oscillator running
19. Pneumothorax can be best determined on HFOV by
a. Auscultation
b. Loss of chest wiggle on the affected side. Confirm with chest x-ray
c. Changes in displayed Paw and Delta-P
20. Focus should be placed on weaning which HFOV parameter first
a. Amplitude
b. Frequency
c. FiO2
21. The following statement best describes weaning large patients from HFOV
a. Patients can be weaned and extubated directly from HFOV
d. Spontaneous breathing is not well tolerated on HFOV and patients should be transitioned to CMV for weaning
c. Weaning should not be attempted until the Amplitude is reduced to
22. Erratic Paw display IS NOT caused by the following
a. Inappropriately low setting of the Paw limit thumbwheel
b. Spontaneous breathing
c. Secretions in the airway
23. If the Max Paw alarm is met, the ventilator will:
a. Depressurize to 12(+3)cmH2O below the Set Max Paw setting,continue to cycle,
providing audible and visual alarms until the fault is resolved.
b. Depressurize to ambient pressure and stop the driver.
b. Continue to run, but visually alarm to alert the user
24. The following statement DOES NOT describe hemodynamic response to HFOV
a. Transient hypotension due to relatively high Paw’s usually responds well to fluid bolus or vasopressors
b. All patients experience hypotension when transitioned from CMV to HFOV
c. Hypotensive patients should be given adequate preload and/or vasopressors prior to transition to HFOV
25. Opacification of lung fields, along with a low SaO2 indicates the following
a. Underinflation, requiring an increase in Paw
b. Overinflation, requiring a decrease in Paw
d. Underinflation, requiring an increase in FiO2
Back