Meconium Aspiration syndrome
Introduction
Meconium aspiration syndrome (MAS) is a common problem that most pediatricians will encounter in the delivery room and normal newborn nursery. Approximately 13% of all live births are complicated by meconium-stained amniotic fluid (MSAF). Fortunately, only 5% of neonates born through MSAF develop MAS .
Definition MAS is defined as respiratory distress in an infant born through MSAF whose symptoms cannot be otherwise explained
Cleary and Wiswell have proposed severity criteria to define MAS:
(1) mild MAS is disease that requires less than 40% oxygen for less than 48 hours,
(2) moderate MAS is disease that requires more than 40% oxygen for more than 48 hours with no air leak, and
(3) severe MAS is disease that requires assisted ventilation for more than 48 hours and is often associated with persistent pulmonary hypertension.
 Causes of meconium-stained amniotic fluid
Causes of meconium-stained amniotic fluid
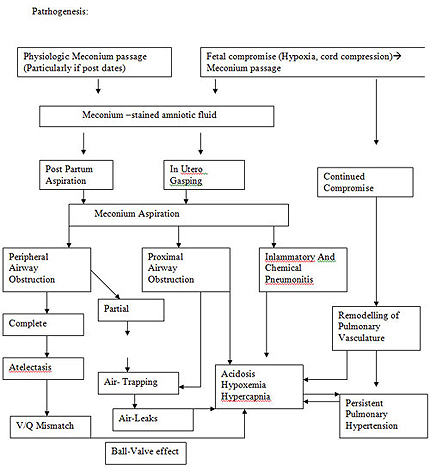
Under normal circumstances, the passage of meconium from the fetus into the amnion is prevented by the lack of intestinal peristalsis, which is caused by several factors, including low motilin levels, tonic contraction of the analsphincter, and a terminal cap of viscous meconium. MSAF may be a naturalphenomenon that neither indicates nor causes fetal distress but simply reflectsa postterm fetus with a mature gastrointestinal tract in which motilin levelshave risen. Vagal stimulation produced by cord or head compression alsomay be associated with the passage of meconium in the absence of fetaldistress. In contrast, meconium passage may occur secondary to an in utero stress, with resultant fetal hypoxia and acidosis producing relaxation of the anal sphincter. Termand postterm neonates are more likely to pass meconium in response to such a stress than preterm neonates. Passage ofmeconium into the amniotic fluid may increase the risk of intra-amniotic infection .
Causes of meconium aspiration syndrome
It is unclear why some infants born through MSAF develop an aspirationsyndrome whereas others do not. Aspiration of meconium may occur inutero or after delivery with the first few breaths. Chronic fetal hypoxiaand acidosis may lead to fetal gasping and the subsequent in utero aspirationof meconium. Mounting evidence suggests that a chronic in utero insult may be responsible for most cases of severe MAS as opposed to an acute
peripartum event . In contrast to these severe cases, the vigorous infant who aspirates meconium-stained fluid from the nasopharynx at birth usuallydevelops mild to moderate disease.
Mechanisms of injury
Meconium seems to be toxic to the lungs in many ways, and it may bedifficult to determine which mechanisms predominate at a given point intime. Mechanisms of injury in MAS are as follows: (1) mechanical obstructionof airways, (2) chemical pneumonitis, (3) vasoconstriction of pulmonary vessels, and (4) inactivation of surfactant.
Mechanical obstruction
Meconium is thick and viscous and may cause complete or partial airwayobstruction. With the onset of respiration, meconium migrates from centralto peripheral airways. Particles of meconium inhaled into the small distalairways cause further obstruction and atelectasis, which lead to areas of unventilated lung with resultant mismatch of ventilation and perfusionwith resultant hypoxemia. Partial obstruction produces a ''ball-valve'' effect in which inhaled air is allowed to enter the alveoli but is unable to escape.
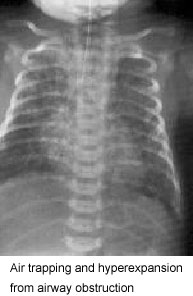
This causes air trapping in the alveoli with further ventilation/perfusion(V/Q) mismatch and may lead to hyperexpansion and air leak syndromes.The risk of pneumothorax is estimated to range from 15% to 33% .
Pneumonitis
Meconium seems to have a direct toxic effect mediated by inflammation.Within hours, neutrophils and macrophages are found in the alveoli, largerairways, and lung parenchyma. The release of cytokines, such as tumor necrosis factor-a, interleukin-1b, and interleukin-8, may directly injure lungparenchyma or lead to vascular leakage, which causes a toxic pneumonitis with hemorrhagic pulmonary edema. Meconium contains many substances, such as bile acids, that, when present in the amniotic fluid, are known to cause direct injury of the cord vessels and amniotic membranes. Some elaborated products also have a direct vasoconstrictive effect on the placental and umbilical cord vessels.
Pulmonary vasoconstriction
Severe MAS may be complicated by persistent pulmonary hypertension.This pulmonary vasoconstriction is, in part, the result of the underlying in utero stressor.The release of vasoactive mediators, such as eicosanoids, endothelin-1, and Prostaglanden E2 (PGE2), as a result of injury from meconium seems to play a role in the development of persistent pulmonary hypertension .
Surfactant inactivation
In the early 1990s, researchers recognized that meconium inactivates surfactant. Meconium displaces surfactant from the alveolar surface and inhibits its surface tension-lowering ability . Studies demonstrated a direct inhibitory effect of meconium on the function of surfactant in vitro and in in vivo animal models . Lung lavage fluid in infants with MAS has shown evidence of known surfactant inhibitors . A full-term baby born with a sufficient quantity of surfactant may develop surfactant deficiency by inactivation that leads to increased surface tension with atelectasis, decreased lung compliance, decreased lung volumes, and resultant poor oxygenation
Risk factors for meconium-stained amniotic fluid _ Maternal hypertension _ Maternal diabetes mellitus _ Maternal heavy cigarette smoking _ Maternal chronic respiratory or cardiovascular disease _ Postterm pregnancy _ Pre-eclampsia/eclampsia _ Oligohydramnios _ Intrauterine growth retardation _ Poor biophysical profile _ Abnormal fetal heart rate patterns
Diagnosis
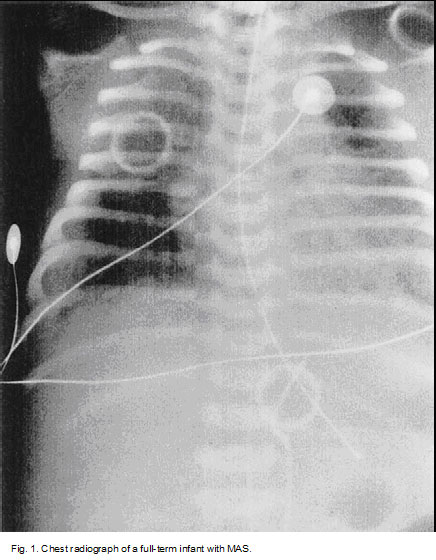
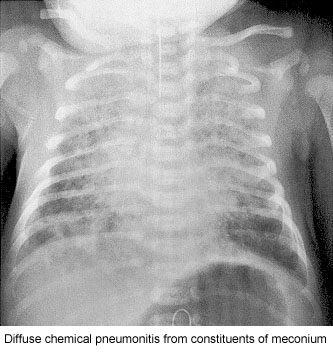
MAS must be considered in any infant born throughMSAF who develop ssymptoms of respiratory distress. The classic roentgenographic findings inMAS are described as diffuse, asymmetric patchy infiltrates, but because ofthe diverse mechanisms that cause disease, various radiographic findings may be present (Fig. 1).
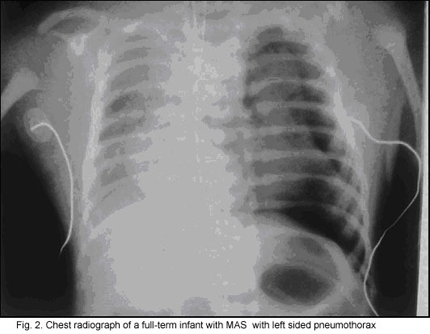
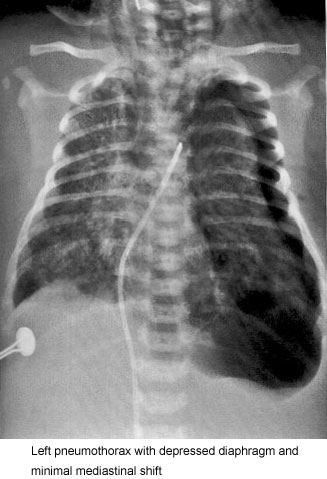
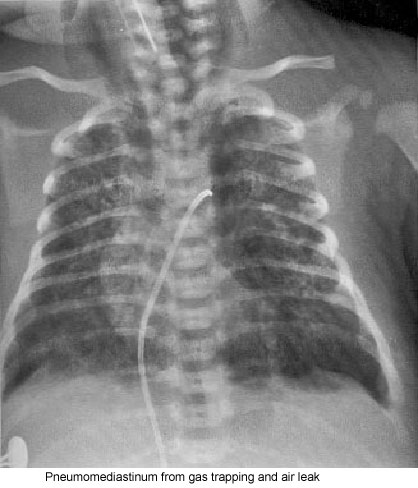
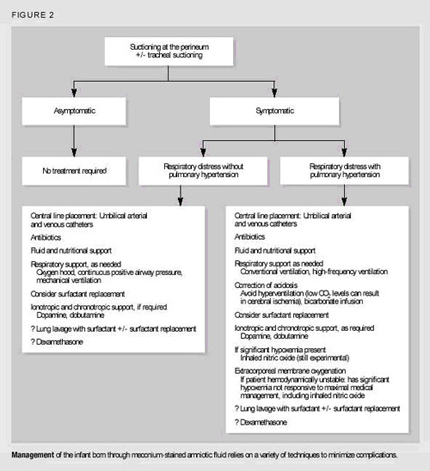
Frequently, overaeration is present, which may leadto air leak syndromes, such as pneumothorax, pneumomediastinum, or pulmonary interstitial emphysema (Fig. 2).
In patients with the classic radiographic findings of MAS, radiographic clearing is slow over a period of days or weeks . A two-dimensional echocardiogram to evaluate for pulmonary hypertension may be useful early in an infant's course.
 Management
Management
The obstetric focus is on the possible need for intervention designed to decrease the risk of MAS. MSAF should be considered a possible warning sign of fetal distress. Many authors recommend that if MSAF is seen, obstetricians should monitor carefully the fetal heart rate tracing and have a low threshold for performing additional testing, such as fetal scalp pH .
A newer modality for monitoring the fetus is fetal pulse oximetry. Fetalpulse oximetry was approved for use by the Food and Drug Administrationin May 2000 and is finding increased acceptance among obstetricians . Inthe case of nonreassuring fetal heart rate patterns, studies have shown a highcorrelation between fetal oxygen saturation below 30% and a scalp pHvalue of 7.2 .
Thus, many had high hopes that utilization of fetal pulseoximetry could identify at risk infants and improve outcomes. Results ofrandomized trials have been mixed.
In a randomized trial of 5341 nulliparous women, use of pulse oximetry did not reduce the rate of Caesarean delivery or improve infant outcomes.
Amnioinfusion has been proposed for interventions when monitoring detectsvariable fetal heart. During this procedure, a sterile isotonic solution is infused into the amniotic cavity via catheter. By adding volume into the cavity, the meconium is diluted. The decreased risk of cord compression may decrease hypoxia and decrease fetal gasping .
Good evidence exists that amnioinfusion is effective at reducing the consistency of the meconium. The procedure also seems to be relatively safe . In trials conducted in obstetric settings with the capability of intensive intrapartum surveillance, few benefits have been found .
However, in settings without these capabilities, often in developing countries, amnioinfusion has been protective with improved neonatal outcomes (as measured by Apgar scores), reduced meconium aspiration syndrome (4% vs. 18%) and improved maternal outcomes .
Accordingly, ACOG Committee on Obstetric Practice has recommended: ''Based on current literature, routine prophylactic amnioinfusion for the dilution of amnioinfusion for meconium-stained amnionotic fluid should be done only in the setting of additional clinical trials. However, amnioinfusion remains a reasonable approach in the treatment of repetitive variable decelerations, regardless of amniotic fluid meconium status.'' .A recent meta-analysis found a 76% reduction in MAS with amnioinfusion .
Intrapartum management
Intrapartum suctioning
Intrapartum suctioning, combined with intubation after delivery, has been considered standard procedure for more than 25 years based on the seminal work of Carson et al . However, only recently has intrapartum suctioning been studied with randomized trials. The goal is to clear as much meconium as possible from the airway before the infant is able to take a breath. Based on the evidence, the ACOG Committee on Obstetric Practice, in September 2007, revised recommendations and recommended that ''all infants with MSAF should no longer receive intrapartum suctioning. If meconium is present, and the newborn is depressed, the clinician should intubate the trachea and suction meconium from beneath the glottis'' . The appropriate pediatric intervention in infants born through meconium- stained fluid depends on whether the infant is ''vigorous.'' Current neonatal resuscitation guidelines define an infant as vigorous if he or she has (1) strong respiratory efforts, (2) good muscle tone, and (3) a heart rate more than 100 beats/min . When this is the case, there is generally no need for tracheal suctioning, and the pediatrician may proceed with routine management
When an infant is not vigorous, the goal is to clear the airway as quickly as possible to minimize the amount of meconium aspirated. The infant may be given free-flow oxygen and placed under a radiant heater, but drying and stimulating should be delayed. At this point, direct laryngoscopy should be performed with suctioning of the mouth and hypopharynx (with a 12F or 14F suction catheter) under direct visualization, followed by intubation and then applying suction (approximately 100 mm Hg) directly to the endotracheal tube as it is slowly withdrawn. The process is repeated until either''little additional meconium is recovered, or until the baby's heart rate indicates that resuscitation must proceed without delay'' .
Postnatal management of the infant
 Approach to the apparently well infant
Approach to the apparently well infant
Most infants born through MSAF require no interventions and may remain with their family in the delivery room. Because MAS does not always present immediately, however, it is important to monitor these infants closely for signs of respiratory distress. Infants at risk for MAS may have signs of postmaturity, such as peeling skin, long fingernails, and yellow stained skin and umbilical cord. One should observe the infant for tachypnea and cyanosis and for grunting, nasal flaring, and retractions. The chest may appear barrel shaped as a result of overinflation due to a ball-valve effect as the meconium plugs the lower airways. Rales and rhonchi may be auscultated . Most infants who develop symptoms do so in the first 12 hours of life.
Approach to the ill newborn
Infants at risk for MAS who show signs of respiratory distress must be transferred to the neonatal intensive care unit. Infants with MAS can deteriorate rapidly and must be monitored closely.
In the neonatal intensive care unit, conventional therapy for MAS is aimed at increasing oxygenation while minimizing the barotrauma that may lead to air leak syndromes. The infant with severe MAS can spiral into a vicious cycle of hypoxemia that leads to acidosis, which together cause pulmonary vein constriction. In its severest form, this condition may lead to persistent pulmonary hypertension. The resultant right-to-left shunting at the level of the ductus arteriosus, the atrial level, or both causes further cyanosis and hypoxemia, which perpetuate the cycle. It is imperative that the baby make a successful transition from intrauterine to extrauterine life, with a drop in pulmonary arterial resistance and an increase in pulmonary blood flow.
The amount of ventilatory support needed for MAS depends on the amount of respiratory distress. Some babies require only an oxygen hood, but approximately 40% of the babies required mechanical ventilation and an additional 10% required continuous positive airway pressure . Hyperventilation with resultant alkalinization decreases pulmonary vascular resistance, but no trials have compared the outcome of MAS with hyperventilation versus a ''gentler'' ventilation strategy. In fact, in an observational study, infants treated with hyperventilation had worse outcomes than those treated with strategies designed to normalize pH and CO2.
Judicious use of PEEP (4-6 cmH20).
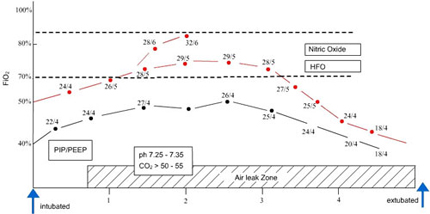
Since meconium aspiration is an obstructive lung disease , time constants for expiration is prolonged in severely involved areas of lung . Attention must be paid to expiratory time ( ventilatory rate) to prevent inadvertent PEEP, further gas trapping ,and alveolar rupture. High-frequency ventilators minimize barotrauma through the use of subnormal tidal volumes at supraphysiologic rates, which allow use of high mean airway pressures without the concurrent use of high peak pressures. High-frequency ventilators may slow the progression of meconium down the tracheobronchial tree and allow more time for meconium removal . No prospective, randomized, controlled trials have compared conventional ventilation versus high-frequency ventilation in MAS.
Surfactant
Native surfactant is inactivated by bile salts present in meconium, and infants with MAS have reduced surfactant synthesis capabilities . Two randomized, controlled trials have evaluated the efficacy of exogenous surfactant therapy in MAS. The results have been promising, with a decrease in the number of infants requiring ECMO and a possible reduction in the risk of pneumothorax . There was, however, no difference in mortality. A Cochrane meta-analysis of four randomized trials confirmed that surfactant replacement showed no effect on mortality, but did significantly reduce the use of ECMO [relative risk 0.64, 95% confidence interval, 0.46, 0.91; number needed to benefit 6] .
Surfactant lavage
In an attempt to remove noxious material from the lungs, minimize obstruction, and simultaneously offset the inactivation of surfactant by meconium,some investigators have examined lung lavage with dilute surfactant. The benefits seem to be an increase in oxygenation and decrease inneed for mechanical ventilation. The procedure often had to be halted because of hypotension or periods of hypoxemia. This is an exciting area of investigation,however, and additional trials are warranted.
Inhaled nitric oxide
Meconium aspiration syndrome is frequently accompanied by persistent pulmonary hypertension. Inhaled nitric oxide causes selective pulmonary vasodilation by acting directly on the vascular smooth muscle. It activates guanylate cyclase and increases cyclic GMP, then binds to hemoglobin and is inactivated. When nitric oxide is delivered as an inhaled drug, it causes selective pulmonary capillary vasodilation with minimal effects on other body systems. By dilating the blood vessels in well-ventilated areas of lung, inhaled nitric oxide decreases the ventilation perfusion ratio mismatch and improves oxygenation in infants with persistent pulmonary hypertension. Seminal trials demonstrated a decreased need for ECMO [RR 0.61, 95%CI 0.51, 0.72], but no difference in mortality .
Proper administration of inhaled nitric oxide requires adequate delivery to the alveoli. In a large, randomized, multicenter trial, infants with MAS responded well to the combination of inhaled nitric oxide and High Frequency Oscillatory Ventilation (HFOV), likely because of improved lung inflation and better delivery of the drug . Inhaled nitric oxide is approvedby the Food and Drug Administration for the treatment of hypoxic respiratoryfailure in term and near-term infants.
Extracorporeal membrane oxygenation
Since the introduction of treatment of persistent pulmonary hypertension with inhaled nitric oxide, the need for ECMO has decreased. Approximately 40% of infants with MAS treated with inhaled nitric oxide fail to respond and require bypass . Compared with other population subsets that require ECMO, infants with MAS have a high survival rate (on theorder of 93%-100%) . It is notable that many pediatricians underestimate this survival rate [50].
 MANAGEMENT
MANAGEMENT
MAS without PPHN
Primary -
1. Temperature skin probe 36.5? C.
2. Metabolic, Na+ K+ , Ca , Glucose
3. Blood pressure - IV fluids , ionotropes
Dopamine 5 - 10 ?g/kg/min ? Dobutamine 5 - 10 ?g/kg/min , to keep the Mean arterial blood pressure on the upper range of normal especially if blood gases indicate intra-pulmonary shunt. FiO2 x 5 = Target PaO2 . In clinical practice FiO2 x 4 = Acceptable PaO2 ( X ) without significant shunt.(i.e. 40% x 4 = 160 mmHg PaO2 if FiO2 0.4). This value minus 15 % of the same is the upper permissible range of PaO2 for that FiO2. Values below this , i.e. X - 15% = significant intra-pulmonary shunt.
ASSISSTED VENTILATION
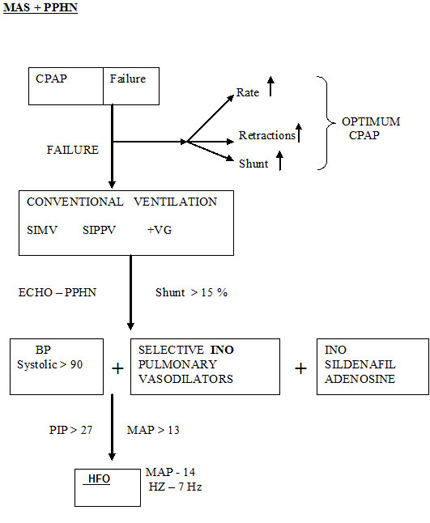
CPAP could be tried as per CPAP protocol .Refer CPAP chapter . Intubation would be for worsening of disease and hypoxemia
OBSTRUCTIVE AIRWAY DISEASE
Start , Initial settings PIP 22 , PEEP 4 , IT 0.35 , Rate 40-50 /min
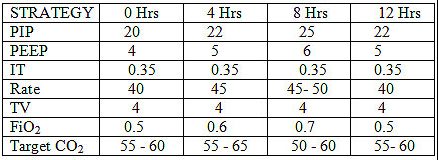
Please refer diagram .Air leaks should be anticipated and treated . Don't change ventilatory pressures for the sake of air leaks. If air leak is progressive tidal volume , PIP and rate may be reduced . Only rarely is IT changed in MAS as it is an air trapping disease and could aggravate air leak. In our unit we do not generally increase beyond 0.35 - 0.4 seconds. The addition of PEEP is beneficial to open up the tracheo-bronchial tree , despite being an air trapping syndrome. The rate has to be reduced as is feasible based on the tidal volume to reduce the air trapping and inadvertent PEEP. An I:E ratio of about 1:2 should be aimed for. If the MAP exceeds 13 or the PIP exceeds 27 , high frequency oscillatory ventilation is a better option.
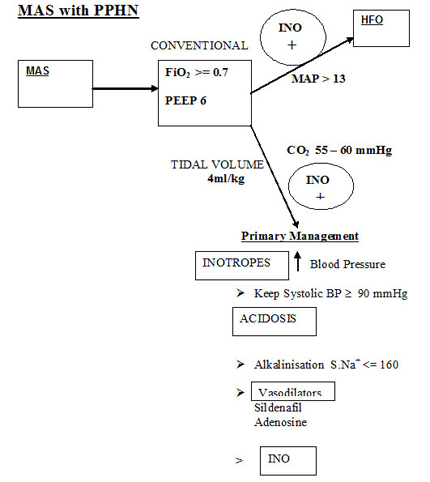
It is imparative to optimise the systemic blood pressure above the pulmonary artery pressure . ECHMO can demonstrate intra-cardiac shunt as R ? L or L ? R. This is the basic approach.
Pressure ventilation has drawback despite widespread use. Volume ventilation with its limitations would be advantageous in this scenario of constantly changing airway resistance due to debris , inflation . spasm and infection. 4 ml/kg is used in our unit. The lung volume have to be optimised if INO or Sildenafil have to work. A target CO2 ? 55 mmHg would be
advisable. This could drop the blod pH so alkalinisationmay have to be considered. In clinical practice fall in CO2 reduces the pulmonary vascular resistance, and eventually the baby's hypoxemia resolves even when sitting on a FiO2 of 1.0 (Weny et al 1985). Consistency and appropriate ventilatory and blood pressure strategy are mandatory. Hypoventilation is to be avoided at all costs and could lead to volutrauma and air leaks. ECHO helps to document reduction in pulmonary hypertension.
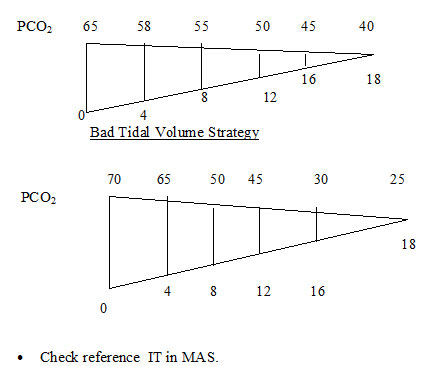
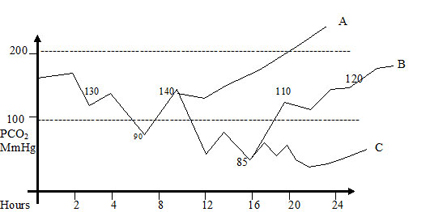
OI AadO2 A- Indicates good strategy of V/Q or mild degree of PPHN. Although to reduce FiO2 within 8 hours of stability of the pulmonary vasculature.
OI AadO2 B - indicates moderately severe disease . Consistency in ventilator management mainly PEEP and not IT or PIP would make the baby eventually oxygenate better. Wrong ventilatory strategy ? PIP or ? IT indiscriminately can lead to lung injury and air leak. Most cases resolve with gentle ventilation and optimum PEEP . A gradual decline in PaCO2 could reduce pulmonary vascular resistance as the lung volume optimises. Vasodilators are indicated early. ECHO diagnosis of degree of PPHN severity of shunt on ABG determines this modality.
OI AadO2 C - This indicates very severe disease. Plan is to maintain PaCO2 60 - 80 mmHg for 24 hours and strategy to "cheat" PPHN by reducing FiO2 as mentioned above. A bold decision has to be taken over the next 48 - 72 hours to wean down FiO2 between0.6 - 0.7 if possible, keeping PaO2 > 50 mmHg. This bold strategy along with vasodilators have helped us wean babies with PaO2 < 100 mmHg in FiO2 1.0 , over a period of 1 upto week. These may be CPAP dependent with FiO2 > 0.6 upto 2 weeks. If the MAP comes close to 8 or 9 , they should be extubated to higher CPAP (6 -7 cm H2O) . On the contrary , if high FiO2 and pressures are maintained more than 24 hours a vicious cycle acute lung injury and hypoxemia would precludes weaning and the baby would behave as a cyanotic heart diease. A cardiac ECHO within a few hours of birth is required before embarking on this bold strategy.
If stability in PaO2 consistently above 100 - 150 mmHg over 8 - 12 hours and narrowing of pulse oximetry (pre and post ) an attempt can be made to reduce the FiO2 at 2 % every half an hour till an FiO2 of 0.9. Then 5% every half an hour till we reach Fio2 0.7. Flip flops occur in this period and to same extent it is the authors contention that babies on "lower " PEEP experience this phemenon ore often than on optimum PEEP. It is imparative to try reducing FiO2 to 0.7, if possible within 8-12 hours to reduce oxygen toxicity and lung injury.
Bibliography
1. Wiswell TE. Advances in the treatment of the meconium aspiration syndrome. Acta Paediatr2001;90(Suppl):28-30.
2. Cleary GM, Wiswell TE. Meconium-stained amniotic fluid and the meconium aspirationsyndrome: an update. Pediatr Clin North Am 1998;45:511-29.
3. Piper HM, Newton ER, Berkus MD, Peairs WA. Meconium: a marker for peripartum infection. Obstet Gynecol 1998;91:741-5.
4. Blackwell SC, Moldenhauer J, Hassan SS, et al. Meconium aspiration syndrome in term neonates with normal acid-base status at delivery: is it different? Am J Obstet Gynecol 2001;184:1422-6.
5. Ghidini A, Spong CY. Severe meconium aspiration syndrome is not caused by aspiration of meconium. Am J Obstet Gynecol 2001;185:931-8.
6. Hageman JR, Caplan MS. An introduction to the structure and function of inflammatory mediators for clinicians. Clin Perinatol 1995;22:251-61.
7. Greenough A. Surfactant replacement therapy for non-respiratory distress syndrome neonatal respiratory disease: research or clinical application? Eur J Pediatr 1995;154:S2-4.
8. Moses D, Holm BA, Spitale P, Liu MY, Enhorning G. Inhibition of pulmonary surfactant function by meconium. Am J Obstet Gynecol 1991;164:4677-81.
9. Cleary GM, Antunes MJ, Ciesielka DA, Higgins ST, Spitzer AR, Chander A. Exudative lung injury is associated with decreased levels of surfactant proteins in a rat model of Meconium aspiration. Pediatrics 1997;100:998-1003.
10. Higgins ST, Wu AM, Sen N, Spitzer AR, Chander A. Meconium increases surfactant secretion in isolated rat alveolar type II cell. Pediatr Res 1996;39:443-7.
11. Dargaville PA, South M, McDougall PN. Surfactant and surfactant inhibitors in Meconium aspiration syndrome. J Pediatr 2001;138:113-5.
12. Findlay RD, Taeusch HW, Walther FJ. Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics 1996;97:48-52.
13. Hachey WE. Meconium aspiration. In: Gomella TL. Neonatology.4th edition. New York: Lange Medical Books; 1999. p. 507.
14. Newman B. Imaging of medical disease of the newborn lung. Radiol Clin NorthAm1999;37:1049-65.
15. Shaw K, Clark S. Reliability of intrapartum fetal heart rate monitoring in the postterm fetus with meconium passage. Obstet Gynecol 1988;72:886-9.
16. Gabbe SG. Meconium stained fluid. In: Obstetrics: normal and problem pregnancies. New York: Churchill-Livingstone; 2002. p. 665-6.
17. Kuhnert M, Seelbach-Gobel B, Butterwegge M. Predictive agreement between the fetal arterial oxygen saturation and fetal scalp pH: results of the German multicenter study. Am J Obstet Gynecol 1998;178:330-5.
18. Bloom SL, Spong CY, Thom E, et al. Fetal pulse oximetry and cesarean delivery. N Engl J Med 2006;335:2195-202.
19. Xu H, Hofmeyr J, Roy C, Fraser WD. Intrapartum amnioinfusion for meconium-stained fluid: a systematic review of randomised controlled trials. BJOG 2007;114:383-90.
20. Cialone PR, Sherer DM, Ryan RM, Sinkin RA, Abramowicz JS. Amnioinfusion during labor complicated by particulate meconium stained amniotic fluid decreases neonatal morbidity. Am J Obstet Gynecol 1994;170:842-9.
21. Wenstrom K, Andrews WW, Maher JE. Amnioinfusion survey: prevalence, protocols and complications. Obstet Gynecol 1995;86:572-7.
22.Fraser WD, Hofmeyr J, Lede R, et al. Amnioinfusion for the prevention of the Meconium aspirtion syndrome. N Engl J Med 2005;353:909-17.
23. Das AK, Jana N, Dasgupta S, et al. Intrapartum transcervical amnioinfusion for meconiumstained amniotic fluid. Int J Gynaecol Obstet 2007;97:182-6.
24. ACOG Committee Obstetric Practice. ACOG Committee Opinion Number 346, October 2006: amnioinfusion does not prevent meconium aspiration syndrome. Obstet Gynecol 2006;108:1053.
25. Carson BS, Losey RW, Bowes WA Jr, Simmons MA. Combined obstetric and pediatric approach to prevent meconium aspiration syndrome. Am J Obstet Gynecol 1976;126:712-5.
26. Committee on Obstetric Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 379: Management of delivery of a newborn with meconium- stained fluid. Obstet Gynecol 2007;110:739.
27 Kattwinkel J, editor. Textbook of neonatal resuscitation. 5th edition. Chicago: American Academy of Pediatrics; 2005. p. 2.6-2.7.
28. Miller MJ, Fanaroff AF, Martin RJ. Respiratory disorders in preterm and term infants. In:Martin AFR, editor. Neonatal-perinatal medicine: diseases of the fetus and infant. St. Louis: Mosby; 2002. p. 1025-8.
29. Wiswell TE, Gannon CM, Jacob J, et al. Delivery room management of the apparently vigorous meconium-stained neonate: results of the multicenter, international collaborative trial. Pediatrics 2000;105:1-7.
30. Taeusch ,Ballard, Gleason .respiratory failure in term Infant ,Avery`s diseases of the Newborn; 8 th edition ,705-722).
31. Hachey WE, Eyal FG, Curtet-Eyal NL, Kellum FE. High-frequency oscillatory ventilation versus conventional ventilation in a piglet model of early meconium aspiration. Crit CareMed 1998;26:556-61.
32. Janssen DJ, Carnielli VP, Cogo P, et al. Surfactant phosphatidylcholine metabolism in neonates with meconium aspiration syndrome. J Pediatr 2006;149:634-9.
33. Lotze A, Mitchell BR, Bulas DI, et al. Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. J Pediatr 1998;132:40-7.
34. El Shahed A, Dargaville P, Ohlsson A, et al. Surfactant for meconium aspiration syndrome in full term/near term infants. Cochrane Database Syst Rev 2007; Jul 18;(3):CD002054.
35. Lam BCC, Yeung CY. Surfactant lavage for meconium aspiration syndrome: a pilot study. Pediatrics 1999;103:1014-8.
36. Wiswell TE, Knight GR, Finer NN, et al. A multicenter, randomized, controlled trial comparing Surfaxin (lucinactant) lavage with standard care for treatment of meconium aspiration syndrome. Pediatrics 2002;109:1081-7.
37. Rais-Bahrami KRO, Seale WR, Short BL. Effect of nitric oxide in meconium aspiration syndrome after treatment with surfactant. Crit Care Med 1997;25(10):1744-7.
38. Kinsella JP, Truog WE, Walsh WF, et al. Randomized, multicenter trial of inhaled nitricoxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertensionof the newborn. J Pediatr 1997;131:55-62
39. Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev 2006 Oct 18;(4):CD000399.
40. Kinsella JP, Neish SR, Shaffer E, Abman SH. Low-dose inhalational nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992;340:819-20.
41. Bernbaum J, Schwartz IP, Gerdes M, D'Agostino J, Coburn CE, Polin RA. Survivors of extracorporeal membrane oxygenation at 1 year of age: the relationship of primary diagnosis with health and neurodevelopmental sequelae. Pediatrics 1995;96:907-13.
42. WalkerGM,Coutts JA, Skeoch C, Davis CF. Paediatricians' perception of the use of ECMOto treat meconium aspiration syndrome. Arch Dis Child Fetal Neonatal Ed 2003;88:F70-1.
Meconium aspiration syndrome Oxygenation of infants who have severe MAS may improve if HFJV is used in combination with surfactant [92]. Similarly, HFOV has improved oxygenation in infants who have severe MAS; the combination of HFOV and iNO may be particularly efficacious [93]. The most compelling evidence is from a randomized trial in which ECMO improved the survival of infants who had MAS with an oxygenation index greater than 40 by 50% [94]. Data from the ELSO registry highlight that approximately 94% of infants who have MAS placed on ECMO survive, and other results suggest that this is not associated with an increased risk of neurologic disability
Back