Persistent Pulmonary Hypertension
 CAUSES OF PPHN
CAUSES OF PPHN
| Sepsis |
Congenital diaphragmatic hernia |
| Pneumonia |
Primary pulmonary hyperplasia |
| RDS |
idiopathic |
| MAS |
cardiac malformations |
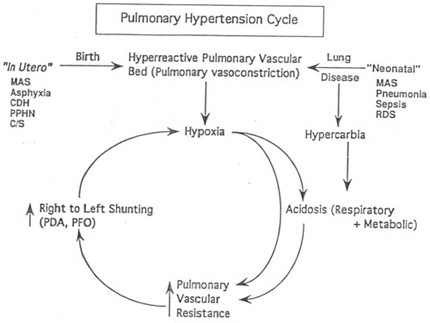
Management of PPHN
(Adapted from Walsh, Sukys M,PPHN, Clin Perinatol 20; 136,1993)
Diagnosis of PPHN
The points enumerated below should make the physician suspect PPHN
. History of Perinatal asphyxia
. Hypoxemia in a newborn out of proportion to the degree of parenchymal lung disease
. Intermittent cyanosis especially to painful stimuli
. Loud single S 2
. Tricuspid incompetence murmur
OBJECTIVE TESTING
Hyperoxia test
Baby is administered 100% oxygen for 5 to 10 minutes. An increase in pao2 > 150 mm Hg excludes most types of cyanotic heart disease and suggests parenchymal lung disease.73 But if the pao2 does increase neither cyanotic heart disease nor PPHN can be eliminated from the differential diagnosis.
Pre and post ductal paO2
A right radial or temporal artery sampling indicating a difference of >=20 mmHg compared with post ductal paO2is considered significant. This occurs only when shunting (R to L) occurs at the ductus and is not seen in atrial shunting across PFO. A pulse Oximetry difference of 5-7% saturation pre and post ductal indicates has the same significance.
Echocardiography
It is a very important critical test in PPHN.
. 2D and color Doppler
. Structural defects of the heart
. Shunting of blood flow (R to L) assessed periodically as medical management continues(PFO and PDA).73
. Right sided systolic time interval>0.5
. Pulmonary artery pressures can be quantified based on tricuspid regurgitation jet of blood Even if there is subsystemic pulmonary artery pressures , medical management by inotropes can show echocardiographic regression of shunt which is the fundamental issue in management of PPHN.
Target systemic B.P > Pulmonary artery B.P
CALCULATING THE SHUNT
Thumb rule FiO2 in oxygen percentage X 4 ==Normal .This is the absolute normal value .
Thumb rule for Intrapulmonary Shunt
Fio2 in oxygen % x 4 = Normal
In clinical practice the intrapulmonary shunt in a normal lung is 5-7%. Pathological intra pulmonary shunt exceeds 15% from normal. e.g. 60 % Fio2 target pao2 is 240 mmHg. If Significant intrapulmonary shunt exists pa02< 200 mmHg.
Hyperoxia Hyperventilation Test
Baby is ventilated with Fi02 1.0 and rate adjusted in order to get C02 between 20 -30mmHg(critical C02).If a rapid improvement in Pa02 is seen(>150 mmHg) a diagnosis of PPHN is supported . 73A negative test does not rule out PPHN. Cardiac disease could be mistaken for PPHN if echocardiography is not done. Echocardiography is the important diagnostic evaluation in the diagnosis of PPHN.
Cardiac Catheterization
This may be important to rule out Total Anomalous Venous Return which cannot be ruled out by 2D echo.
Indices of severity of disease
(1) [Alveolar- Arterial gradient] pA02 - pa02
(ALVEOLAR ) pA02 = [PB - 47] Fi02 - paC02 / R
= [760 - 47] x1 - 40 / 0.8
R = respiratory quotient
PB = barometric pressure
Fi02 = 1.0(severe disease).
(2) Oxygenation index (OI) = MAP x Fi02 x 100
Post ductal pa02
MAP- Mean air way pressure.
Fi02 = 1.0(severe disease).
Grading of Oxygenation index (OI):
OI > 15 - severe respiratory failure
OI > 30-35 - failure to respond to existing mode of ventilatory support.
OI > 40 - ECMO
Oxygenation index may be more useful than (pA02 - pa02) since the former reflects barotrauma also.
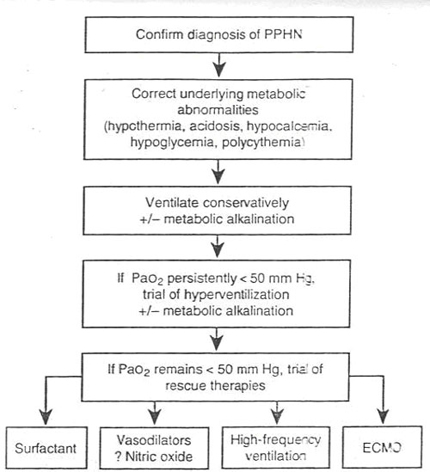
TREATMENT OF PPHN
. The main objective will be to reduce pulmonary artery pressure either pharmacologically or through ventilation ( pCO2 , pH ).
. Systemic blood pressure may have to be increased to beyond levels considered physiological (systolic BP > 90 mm Hg) to show echocardiographic regression of R to L shunt
Systemic blood pressure
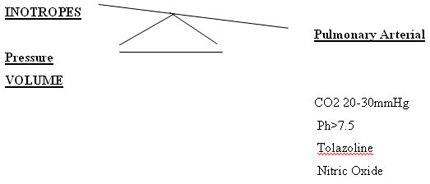 STRATEGIES TO REDUCE PULMONARY ARTERY PRESSURE
STRATEGIES TO REDUCE PULMONARY ARTERY PRESSURE
(1)Hyperventilation
The age old principle of management of PPHN depended upon achieving a low C02 value usually between 20 and 30 mm Hg (some times less than 20 mm Hg ) when rapid improvement in oxygenation occurred ( CRITICAL C02) This usually coincided with a pH > 7.5 due to respiratory alkalosis . 69,70 In severe lung disease excessive PIP and rate may have to be applied to get the critical C02 leading to barotrauma and air leaks..
More sinister is the finding that babies with low CO2 levels especially below20mm Hg exhibited low psychomotor developmental test scores and sensorineural hearing loss. Hearing loss was more likely in babies who had CO2 < 15 mm Hg. 74,75 High frequency ventilation could obviate the need for high pressure and barotrauma but the excessive falls in CO2 during HFO could be disastrous for neurodevelopmental outcome.76,77,78
Current medical practice is not in favour of hyperventilation especially PCo2 less than 30.This modality of treatment is currently not advocated
Conventional ventilation
Wung and workers. have had reasonable results with conservative management. Keeping paO2 50 - 70 mm Hg and pCO2 40 - 60 mm Hg. These babies did not show any long-term sequelae.
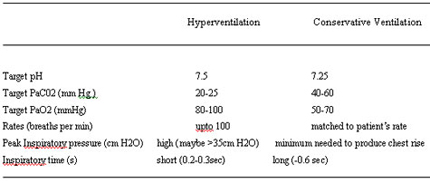
TABLE 9
Management Goals In An Aggressive Hyperventilation Approach And A More Conservative Ventilator Approach In Persistent Pulmonary Hypertension Of The Newborn
Metabolic Alkalanisation It can be done using sodium bicarbonate or tromethamine (THAM) infusion to keep pH> 7.5 –7.6 range. During bicarbonate therapy Na + levels in serum could be close to 160. Here the Na+ levels have to be brought down very slowly to prevent central nervous system dysfunction. Refer (alveolar gas equation) where FiO2=AIR. Currently metabolic alkalanisation has fallen out of favour in neonatal practice.
Vasodilator therapy
The review of literature in the last 10 years has evidenced drugs* mentioned here, which depress systemic blood pressure along with fall in pulmonary arterial pressure. This upsets the fine balance of R L shunt in PPHN, which will be exaggerated, leading to worsening of disease. The ideal vasodilator in PPHN should selectively dilate only the pulmonary vasculature.eg nitric oxide.
TABLE 10
Tolazoline hydrochloride an age-old drug could still show some response in 20 - 30 % cases even though it depressed systemic blood pressure. It is an alpha-adrenergic blocking agent and some of its vasodilator effects are mediated by histamine1 and histamine 2 receptors. It can cause gastrointestinal hemorrhage, systemic hypotension, oliguria, tachycardia and rash.80
Give volume expanders like 10 –20 ml/kg plasma before initiating treatment. Dopamine 2-10 microgram/min and/or dobutamine 5-10 microgram/kg/min should be started before Tolazoline therapy.
.Scalp vein.. or..Right Arm veins should be used.bolus 1-2 mg/kg over 10 mins followed by 1-2 mg/kg/ hour is standard management. Oliguria should be watched for. Tolazoline is a drug that has fallen out of favour in clinical practice due it’s adverse side effects.
INOTROPIC GUIDELINES IN PPHN81,82
TABLE11
High frequency ventilation when MAP > 13 or PIP >27 mm Hg start HFO with MAP + 2 above what is obtained on IMV (high lung volume recruitment strategy.
Nitric oxide
Protocol 1:Start at 20ppm and if good oxygenation id evidenced (PaO2> 150) the Nitric Oxide must be weaned to 5ppm by 24 hour and stopped by 96 hours.
Protocol 2; The base line Nitric oxide dose is increased from 10 to 20 to 40 ppm. The NO is administered for 10 minutes and then turned off for 10 minutes with blood gas obtained before and after each administration. The patient can remain on the lowest dose at which a positive response is seen. Blood methhaemoglobin should be checked 4-6 hourly at any dose above 40 ppm.(detailed discussion in following chapter). Nitrogen dioxide must also be checked continuously at the T-piece of the ventilator circuit.83
HFO + NITRIC OXIDE VERY EFFECTIVE IN PPHN
SILDENAFIL CITRATE it is a selective 3,5 alpha phosphodiasterase inhibitor. This is the principle mechanism of degradation of 3,5 CAMP which causes smooth muscle relaxation of pulmonary vasculature. Inhibition of this Phosphodiesterase prevents degradation of 3,5,CAMP that maintains pulmonary arteriolar dilatation.84,85
ALGORITHMIC APPROACH TO PPHN
Historical events 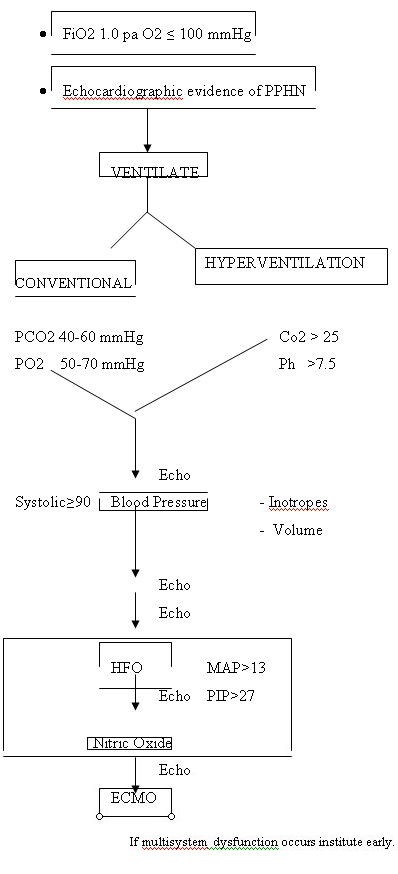
(Echocardiography is done periodically to assess the resolution of pulmonary hypertension and the degree of right to left shunt.)
It also assists to ascertain at what elevation of systemic blood pressure (Increments in inotrope dose) causes regression of the right to left shunt.
MEDICAL MANAGEMENT
AVOID:
hypoxia Ideally keep saturation >100
Hypotension BP systolic> or = 90 mm Hg
Hypothermia skin temp 36.5 degree C
Acidosis blood gas pH> 7.5
Stimulation no painful stimuli, unnecessary suction
Pulmonary vasodilatation
Maximize Fio2 1.0 during acute phase
Cardiac output and blood pressure
Volume 10ml/kgplasma FFP ideally (colloids)
Inotropes- dopamine 2-10 microgram/kg/min (can go upto 15)
Dobutamine5-10 microgram/kg/min
Analgesia and sedation
Analgesia morphine 20-40 microgram/kg/Hour
Fentanyl
Sedation- lorazepam
Midazolam
Phenobarbitone
Paralysis Pancuronium
Vecuronium
Management strategy
1)Optimization of lung volume
2)Pulmonary artery dilatation
• Nitric oxide 6-20 ppm
Sildenafil citrate 1-1.5 mg/kg/dose q4 hourly- 6 hourly oral ng tube
On a conventional ventilator PIP and PEEP are increased to achieve p CO2 >25 mm Hg. This can also be achieved at lower PIP using fast ventilator rates close to 60breaths per minute. PEEP is generally 4-5 cm H2O. It will be extremely important to keep the tidal volume between 6-8 ml/kg while adjusting PIP. Once a paO2>= 100-150mmHg a gradual and steady weaning down of Fio2 is done till it reaches 0.7. Then the pulmonary vasculature is not labile.
Then PIP is to be decreased gradually accepting CO2 40- 50 mmHg and where Fio2 is around 0.4 the PEEP can be reduced from 5 cm h2o to 4 cm H2O. When weaning down pressures towards Day 3 of life a watch has to be kept for development of pulmonary edema. This may be due to excess fluids administered on Day 1 of life to improve blood pressure and perfusion which may re enter the intra vascular space and predispose to pulmonary edema. Once the baby reaches an Fio2 < 0.3 to 0.4 and PIP 18 and PEEP 4 cm H2O extubation has to be considered. The Pneumonitis which develops after the first day has to be managed with appropriate antibiotics.
VENT STRATEGY FAST RATE > OR EQUAL TO 60
OBTRUCTIVE ADEQUATE I:E RATIO
AIRWAY DISEASE SHORTER IT 0.35-0.4
PEEP 4-5 cmsH20
• Use a longer IT (0.4 sec’s) and PEEP (5cm H2O) only if oxygenation is a problem. In our center if MAP >13 and PIP > 27 cms H2O we convert to HFO with MAP of 15 and delta P (amplitude) 45 -75 based on chest movement.
Babylog 8000 and Infant star HFO has limitations on amplitude and hence may not be effective in treating larger babies with MAS. Sensormedics and SLE 2000 HFO+ are powerful oscillators, which can be used on larger babies.
“TIME TABLE FOR PPHN” 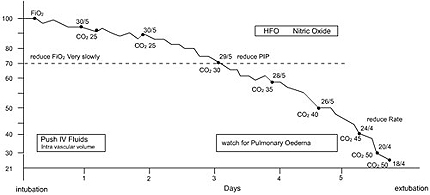
FOLLOW UP
Neuro developmental follow up is required for psychomotor delay and sensori neural hearing loss. Hyperventilation with Co2 levels less than 25 mmHg should definitely be avoided.
S I L D E N A F I L C I T R A T E
INTRODUCTION:
Persistent pulmonary hypertension of the newborn (PPHN) is a complex syndrome with multiple causes, and survivors have high morbidity in the forms of neurodevelopmental and audiological impairment, cognitive delays, and a high rate of rehospitalization. The optimal management still remains controversial. Inhaled nitric oxide (iNO) is currently regarded as the gold standard therapy, but 30% of cases fails to respond,. A number of recent studies have suggested a role for specific phosphodiesterase (PDE) inhibitors in the management of PPHN. Sildenafil, a specific PDE5 inhibitor, appears the most promising of such agents Pulmonary hypertension, is characterized by an increase in pulmonary vascular resistance, resulting in a right-to-left shunt with deoxygenated blood flow through the ductus arteriosus or foramen ovale.1
sildenafil, is a phosphodiesterase-5 inhibitor which increases the concentration of cyclic guanosine monophosphate (cGMP) and causes pulmonary vasodilation.2
Severe pulmonary hypertension is characterised by extensive remodelling of the pulmonary vasculature, with consequent deleterious hypertrophic changes in the right ventricle
Introduction: Incidence of Pulmonary arterial hypertension in neonates is approximately 1.9 per 1000 live births. Despite optimum ventilatory management and haemodynamic support it still remains serious and life threatening condition with high mortality. Sildenafil, a selective phosphodiestrase V inhibitor, is said to be a promising drug in the Persistent Pulmonary Hypertension of Newborn with out untoward complications
Objective of the study: To study the outcome and adverse effects in term neonates with PPHN who received oral Sildenafil in our NICU and their 5-year follow-up.
Materials and Methods: It is a prospective study of eleven neonates who received oral Sildenafil for pulmonary hypertension during the period June 2002 to Jan 2003. Sildenafil was given after echo diagnosis of PPHN and OI->25, at the dose of 1 mg/kg/dose3 every 6th hourly for 3 days through orogastric tube. Acute complications during treatment were noted. A comprehensive Neurological assessment by the pediatric neurologist and Visual (VEP) and hearing assessment (BAER) was done at discharge, Protocolised discharge follow-up up to 5 years was done.
Results: Out of 11 babies’ male and females were 54.5%and 45.5% respectively. Mean MAP and O.I were 19.45 and 32.5 respectively before the use of the drug. It has been found to be reduced to 16.9 and 17.5 respectively. Acute complications during therapy; 5(45.4%) babies were noted to have tachycardia but no other adverse effects like hypotension, rashes, diarrhoea, urinary retention, abnormal LFT or hematological parameters were noted. None of the babies have shown neurodevelopmental, hearing or visual impairment. A tendency towards obesity was observed in 36%.
Conclusions: Sildenafil therapy for PPHN in neonates is not associated with an increase in neurodevelopmental impairment or hearing/visual loss at 5 years of postnatal age
Advantages:
selective pulmonary vasodilator
no effects on systemic arterial pressure,
enhances the effects of inhaled nitric oxide
potent inhibitor of phosphodiesterase-5, relaxes the smooth muscle of the vascular wall
aim
to stabilize the cardiovascular function,
improve oxygenation and
reduce pulmonary arterial pressure
. Treatment
Oral treatment: 2 mg/kg once every 4 hours.
.
IV treatment: Start by giving 200 micrograms/kg IV over 2 minutes., but a continuing infusion of 100 micrograms/kg per hour has sometimes been used.
solution preparation: 50-mg tablet. Dilute in 10 ml DW (1ml=5mg)
Other uses
treatment of pulmonary arterial hypertension secondary to bronchopulmonary dysplasia13.
congenital diaphragmatic hernia..15
Recent studies endothelin receptor antagonists bosentan17and sitaxsentan18 safer, more effective or even complementary to sildenafil.
Mechanism of Action
mechanism involves release of nitric oxide (NO)
NO then activates the enzyme guanylate cyclase,
results in increased levels of cyclic guanosine monophosphate(cGMP), causes smoothmuscle relaxation,and enhances the effect of nitricoxide (NO) by inhibiting phosphodiesterase type 5 (PDE5), which is responsible for degradationof cGMP in the smooth muscle.,inhibition of PDE5 by sildenafil causes increased levels of cGMP,resulting in smooth muscle relaxation sildenafil is selective for PDE5.
Types PDE2, PDE3, PDE4, PDE6, PDE7, PDE8, PDE9, PDE10, and PDE11). The PDE3 isinvolved in control of cardiac contractility.
PDE6, is involved in the phototransductionpathway of the retina. PDE5 is also found in lower concentrations in other tissues including platelets, vascular and visceral smooth muscle, andskeletal muscle
Pharmacokinetics and Metabolism
Sildenafil and its circulating N-desmethyl metabolite are both approximately 96% bound to plasma proteins..
Metabolism and Excretion: Sildenafil is cleared predominantly by hepatic. metabolism (mainly cytochrome P450).
After either oral or intravenous administration, sildenafil is excreted as metabolites predominantly in the feces (80%) and to a lesser extent in the urine (13%) terminal half live - 4 hours.
Interactions:erythromycin, ketoconazole,itraconazole cimetidine, increase plasma levels of sildenafil
Absorption and Distribution:
sildenafil is rapidly absorbed after oral administration, with bioavailability of about 40%.
starts acting 15 minutes after administration
Maximum plasmaconcentrations reach within 30 to 120 minutes (median 60 minutes).
Side effects
1.headache,
2.dizziness,
3.flushing of the face,
4.dyspepsia,
5.nasal congestion
6. transient, impairment of color discrimination
7. transient hypotension 14 1-2 hours after dosing,
8. retinopathy of prematurity (ROP)
9.decrease in hearing
10Rash
photosensitivity reaction, allergic reaction,
tachycardia, hypotension,
Digestive: vomiting, , gastritis, ,
hypoglycemic reaction, hypernatremia.
Nervous hypertonia, , tremors
sweating
1.Weinberger B, Weiss K, Heck DE, Laskin DL, Laskin JD. Pharmacologic therapy of persistent pulmonary hypertension of the newborn. Pharmacol Ther. 2001;89:67-79.
2. Crespo-Martinez C, Morales LV, Alonso RH, Alonso OB, Molero GR. Primary pulmonary hypertension and its management. Farm Hosp. 2004;28:48-55.
3.Carroll WD, Dhillon R. Sildenafil as a treatment for pulmonary hypertension. Arch Dis Child. 2003;88:827-8.
4.Abrams D, Schulze-Neick I, Magee AG. Sildenafil as a selective pulmonary vasodilator in childhood primary pulmonary hypertension. Heart. 2000;84:e4.
5.Travardi JN, Patole SK. Phosphodiesterase inhibitors for persistent pulmonary hypertension of the newborn: a review. Pediatr Pulmonol. 2003;36:529-35.
6.Ghofrani HA, Wiedeman R, Rose F, Olschewski H, Chermuly RT, Weissmann N, et al. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann Intern Med. 2002;136:515-22.
7. Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension. Comparison with inhaled nitric oxide. Circulation. 2002;105:2398-403.
8. Tripathi A, O’Donnell NP. Branch retinal artery occlusion; another complication of sildenafil. Br J Ophthalmol 2000; 84: 934–5.
9.British Journal of Ophthalmology 2004;88:306-307
© 2004 BMJ Publishing Group Ltd 10paris G, Sponsel WE, Sandoval SS, et al. Sildenafil increases ocular perfusion. Int Ophthalmol 2001;23:355–8.[CrossRef][Medline]
11 : Prog Retin Eye Res. 2002 Sep;21(5):485-506Laties A, Zrenner E. Department of Ophthalmology, Scheie Eye Institute, University of Pennsylvania Medical School, Myrin Circle, 51 N 39th Street, Philadelphia, PA 19104, , USA. 12 Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study.Baquero H, Soliz A, Neira F ‘, Sola A. : Pediatrics. 2006 Apr;117(4):1077-83
13 Oral Sildenafil for Treatment of Severe Pulmonary Hypertension in an Infant Kam-lun Ellis Hona, Kam-lau Cheunga, Kiu-lok Siub, Ting-fan Leunga, Man-ching Yama, Tai-fai Foka, Pak-cheung Nga aDepartment of Paediatrics, Prince of Wales Hospital, Chinese University of Hong Kong, Shatin, and bDepartment of Paediatrics, Queen Elizabeth Hospital, Kowloon, Hong Kong, SAR, ChinaBiology of the Neonate 2005;88:109-112 (DOI: 10.1159/000085646)
14 Severe persistent pulmonary hypertension of the newborn in a setting where limited resources exclude the use of inhaled nitric oxide: successful treatment with sildenafil. Juliana AE, Abbad FCDelfzicht Ziekenhuis, Jachtlaan 50, 9934 JD , Delfzijl, The Netherlands , amadu@gmx.net.
15.Combination pharmacotherapy for severe neonatal pulmonary hypertension.Filan PM, McDougall PN, Shekerdemian LS Departments of Neonatology, Royal Children's Hospital, Melbourne, Australia. Published 24 April 2006 in J Paediatr Child Health, 42(4): 219-20.
16.Prasad S, Wilkinson J, Gatzoulis MA. Sildenafil in primary pulmonary hypertension. N Engl J Med 2000;343:1342
17• Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002;346:896–903
18• Barst RJ, Rich S, Widlitz A, et al. Clinical efficacy of sitaxsentan, an endothelin-A receptor antagonist, in patients with pulmonary arterial hypertension. Chest 2002;121:1860–8
Cyanosis, derived from the Greek word kuaneos meaning dark blue, refers to the bluish discoloration of the skin, nailbeds, or mucous membranes. If cyanosis is limited to the extremities, it is referred to as acrocyanosis or peripheral cyanosis. This is relatively common in young infants and is generally a physiologic finding owing to the large arteriovenous oxygen difference that results during slow flow through peripheral capillary beds. In contrast to acrocyanosis, central cyanosis is present throughout the body and is evident in the mucous membranes and tongue. Central cyanosis indicates the presence of potentially serious and life-threatening disease, and requires immediate evaluation. The clinician will need to rapidly consider respiratory, central nervous system, hematologic, cardiac, and metabolic causes [1].
It is often a challenge to define an optimal PaO2 in the newborn with mild cyanosis or respiratory distress. The focus should not necessarily be on achieving an exact number, but rather on avoiding tissue hypoxia by providing adequate oxygen transport to the body tissues. Oxygen is carried in the blood in 2 forms. Virtually all of the oxygen content in the blood is that carried by hemoglobin: each gram of hemoglobin can combine with 1.34 mL of oxygen. In contrast, the amount of oxygen dissolved in the plasma (0.003 mL per 100 mL of plasma) is clinically insignificant. Therefore, the goal should be to achieve adequate hemoglobin saturation and perfusion of the tissues.
While oxygenated hemoglobin is bright red, reduced hemoglobin is dark blue or purple in color and is what produces the dusky or blue color of the skin and mucous membranes. An important concept is that cyanosis is dependent upon the absolute concentration of reduced hemoglobin, rather than on the oxygen saturation or the ratio of reduced hemoglobin to oxyhemoglobin [2]. With careful observation, cyanosis may become apparent when the deoxygenated hemoglobin content is as little as 3 g per 100 mL. Therefore, infants with polycythemia may exhibit cyanosis at relatively high arterial saturations, whereas it is more difficult to discern cyanosis in a severely anemic infant unless the oxygen saturation is extremely low (Table 1). In general, the relatively high hemoglobin of the normal infant tends to facilitate the recognition of cyanosis. At the same time, the relative concentration of fetal hemoglobin and its unique characteristics of oxygen binding need to be considered, as these factors may impair the recognition of cyanosis. The ratio of fetal to adult hemoglobin varies from infant to infant, and the proportions of each hemoglobin affect the oxygen saturation resulting at any given PaO2. Thus, if a baby has mostly adult hemoglobin, central cyanosis (arterial saturation, Division of Neonatology, Children's Memorial Hospital and Northwestern University, Chicago, IL.
Reprint requests and correspondence: Robin H. Steinhorn, MD, Division of Neonatology, Children's Memorial Hospital and Northwestern University, 2300 Children's Plaza #45, Chicago, IL 60614. (E-mail: r-steinhorn@northwestern.edu) 1522-8401/$ – see front matter C 2008 Elsevier Inc. All rights reserved. 169 doi:10.1016/j.cpem.2008.06.006
75% to 85%) will be observed when the PaO2 falls below 50 mm Hg. In contrast, if the baby has mostly fetal hemoglobin, central cyanosis may not be observed until the PaO2 drops well below 40 mm Hg. Thus, infants with a high proportion of fetal hemoglobin may have a serious reduction in oxygenation before cyanosis is clinically apparent (Figure 1).
Normal Cardiopulmonary
Adaptation at Birth
At birth, profound changes in the cardiovascular and respiratory systems occur to allow the infant to adapt to air breathing. An understanding of these normal transitional changes is important in evaluating a cyanotic infant, as their disruption is likely to lead to cyanosis [3]. The fetus has a unique circulatory pattern because the placenta, not the lung, serves as the organ of gas exchange. Less than 10% of the cardiac output is circulated through the pulmonary vascular bed, and the placenta, as the organ of gas exchange, receives nearly half of the cardiac output. Pulmonary blood flow remains low owing to elevated pulmonary vascular resistance; pulmonary pressure is equivalent to systemic pressure because of active vasoconstriction. Fetal circulation shunts blood from the right to the left atrium across the foramen ovale and from the pulmonary artery to the descending aorta through the ductus arteriosus providing direct blood flow to the placenta.
The fetal lungs produce fluid that fills the alveoli, bronchi, and trachea. At birth, catecholamines and other hormones that increase during labor cause a rapid switch from net secretion to net absorption of liquid in alveolar spaces. As the lungs fill with air, lung fluid is removed via the trachea and absorption by the pulmonary capillaries and lymphatics. Pulmonary vascular resistance drops dramatically and permits an 8- to 10-fold rise in pulmonary blood flow. Simultaneously, the low vascular resistance bed of the placenta is removed and systemic vascular resistance increases. As pulmonary pressure falls to less than systemic pressure, pulmonary blood flow increases and blood flow through the patent ductus arteriosus reverses direction. Functional closure of the ductus occurs over the first several hours of life, largely in response to the increased oxygen tension. Left atrial pressure also increases leading to closure of the foramen ovale. These events therefore eliminate the fetal right to left shunts and establish the normal postnatal circulatory pattern of pulmonary and systemic circulations. Within 24 hours after birth, pulmonary artery pressure typically decreases to approximately 50% of mean systemic arterial pressure and continues to drop over the next 2 to 6 weeks until adult values are attained.
TheABCsofDifferentialDiagnosis
in the Cyanotic Infant
Using an airway, breathing, circulation (ABC) algorithm of evaluation will allow the emergency department (ED) practitioner to systematically consider the most common causes of cyanosis in the newborn period (Table 2). A: Airway-Upper and Lower Airway Disease
Although airway conditions will generally present shortly after birth, these should be considered during the initial evaluation of the infant presenting to the ED with respiratory distress [4]. Choanal atresia occurs in ~1:5000 infants, with unilateral disease being more common. Choanal atresia should be suspected when an infant's distress is more obvious in a quiet state and Table 1 Effect of hemoglobin concentration on the recognition of cyanosis.
Hgb (g)
Reduced
Hgb (g) Sao2
Total Hgb-reduced
Hgb/total Hgb
20 3 85 (20-3)/20
8 3 62 (8-3)/20
These data show the expected levels of saturation for 2 hypothetical babies: one with a normal hemoglobin (20 g/dL) vs one with a low hemoglobin (8 g/dL). Both babies have a fixed reduced hemoglobin level of 3 g. The infant with the hemoglobin of 20 will have a saturation of 85%and may appear cyanotic. However, the severely anemic infant may not appear cyanotic until the saturation is critically low. Figure 1 Representation of the different characteristics of oxygen binding in fetal vs adult hemoglobin. For a hypothetical PaO2 of 45, the saturation of adult hemoglobin would fall below 80%, typically creating a cyanotic appearance. However, the binding characteristics of fetal hemoglobin would allow for the saturation to remain in the mid-80s, which may not be associated with overt cyanosis. 170 R.H. Steinhorn
improves during crying. It can be confirmed by the inability to pass a suction catheter through the nares into the oropharynx, as well as via medical imaging, with computed tomography scanning currently serving as the radiographic procedure of choice. Placement of an oral airway should provide immediate improvement. Other associated anomalies are very common; in particular, CHARGE sequence (coloboma, heart disease, atresia of choana, retarded growth and development, genitourinary anomalies, ear/hearing anomalies) should be considered. Micrognathia, retrognathia, or the Pierre Robin sequence generally presents early in life and will be obvious on physical examination. The airway obstruction from the posterior tongue is more pronounced in the supine position. When present, a cleft palate does not cause respiratory distress unless feeding difficulties are severe. These infants may require tracheostomy for several years until the mandible grows enough to maintain the tongue in a more anterior position.
Laryngomalacia is a congenital abnormality of the larynx and is the most common cause of inspiratory stridor in infants. Although it may be noted immediately after birth, it commonly presents at several weeks of age. Airway symptoms typically worsen with crying, feeding, and respiratory infections. Gastroesophageal reflux is a common association. Subglottic stenosis may occur as a congenital malformation or be acquired after prior airway manipulation. Infants present with stridor, respiratory distress, or obstructive apnea.
Vocal cord paralysis may be associated with birth or surgical trauma and is another common cause of stridor in the newborn. It is typically unilateral, causing a hoarse cry and minimal respiratory symptoms. In contrast, bilateral vocal cord paralysis can cause severe respiratory distress, and a tracheostomy may be required. In these cases, central nervous system anomalies such as the Arnold-Chiari malformation should be considered.
Other conditions may cause intrinsic or extrinsic compression of the trachea. Tracheal stenosis is characterized by expiratory stridor, respiratory distress, wheezing, or persistent cough. Symptoms typically worsen after an upper airway infection. The diagnosis is confirmed by direct bronchoscopic visualization. Tracheal stenosis is often associated with complete tracheal rings, which may require extensive surgical repair in case of multiple rings or long segment stenosis. A number of conditions may produce extrinsic airway compression. Vascular rings and slings caused by abnormal development of mediastinal vessels can compress or deviate the trachea causing airway obstruction. An anomalous distal origin of the innominate artery from the aortic arch is the most common cause, but other anomalies include double aortic arch or an aberrant right subclavian artery. Specialized cardiac computed tomography or magnetic resonance imaging studies are
helpful in accurately defining the anatomy. Neck or mediastinal masses such as teratomas and cystic hygromas represent large lesions that can cause extrinsic compression of the trachea; these are typically associated with visible neck masses. Subglottic hemangiomas should be considered in infants that have skin hemangiomas. As hemangiomas typically increase in size over the first 6 to 12 months of life, symptoms often emerge after an initially benign history.
B: Breathing—Lung Disease
Neonatal pneumonia is most commonly acquired at the time of birth and usually causes diffuse rather than lobar infiltrates. The initial radiograph is frequently indistinguishable from the ground glass appearance of respiratory distress syndrome, although pleural effusions are more characteristic of pneumonia. Bacterial pneumonia is most common, and frequent pathogens include group B ß-hemolytic streptococci and Gram-negative Table 2 Causes of cyanosis.
A B C
Airway Breathing Circulation
Choanal atresia Pneumonia Oxygen carrying capacity Micrognathia Congenital diaphragmatic hernia Polycythemia Pierre Robin sequence Congenital cystic adenomatoid malformation Anemia Laryngomalacia Pulmonary sequestration Methemoglobinemia Vocal cord paralysis Congenital lobar emphysema Congenital heart disease Tracheal stenosis Pulmonary hypoplasia Decreased pulmonary blood flow Vascular slings/rings Phrenic nerve palsy Tricuspid atresia Cystic hygroma Hypoventilation Pulmonary atresia Hemangioma Pulmonary stenosis
Other neck masses Tetralogy of Fallot Ebstein’s anomaly
Inadequate mixing
Transposition of the great arteries Persistent pulmonary hypertension Evaluation and management of the cyanotic neonate 171 enteric bacilli (Escherichia coli, Klebsiella, Enterobacter). Important elements of the maternal history will include colonization with group B ß-hemolytic streptococci with or without adequate intrapartum prophylaxis (N2 doses of penicillin before delivery), as well as a history of prolonged rupture of membranes (N18 hours), or a history of maternal fever or chorioamnionitis. Herpes simplex and cytomegalovirus are viral causes of neonatal pneumonia, but typically present as components of disseminated infections. Congenital chlamydia infections can cause pneumonia that presents between 2 and 8 weeks of age, typically with upper respiratory symptoms associated with a cough and apnea.
Congenital lung abnormalities are rare but important causes of respiratory distress in the newborn [4]. In many cases, infants are initially asymptomatic, with respiratory distress developing over time. Careful review of the chest x-ray should reveal these lesions. Congenital diaphragmatic hernia is a relatively common birth defect, but because of its frequent association with significant pulmonary hypoplasia and significant pulmonary hypertension, it almost always presents shortly after birth.
Congenital cystic adenomatoid malformations are extremely rare lung abnormalities composed of cystic lung tissue with communication to the bronchial tree. Medical imaging will help to differentiate this lesion from a congenital diaphragmatic hernia. Pulmonary sequestration is a rare condition characterized by nonfunctioning primitive lung tissue that does not communicate with the tracheobronchial tree and receives vascular supply from the systemic circulation (thoracic or abdominal aorta). Sequestrations may occasionally present in the neonatal period with signs of congestive heart failure owing to the “run-off” circulation, but more commonly present later in life with recurrent infections. Congenital lobar emphysema is an overinflated, hyperplastic area of the lung surrounded by otherwise normal lung tissue. These are most common in the upper lobes. Symptoms are typically progressive, but may be rarely present at birth. Surgical excision is usually curative, although overinflation of remaining lung areas can occur.
It is important to remember that respiratory failure and cyanosis may occur secondary to other organ system dysfunction. For instance, birth injury associated with neurologic depression or hypoxic-ischemic encephalopathy is commonly associated with hypoventilation.
Phrenic nerve injury may cause diaphragmatic paresis. In addition, excessive oral secretions and inadequate swallowing may obstruct the airway and cause respiratory distress. Hypoglycemia may cause central nervous system depression and secondary respiratory distress; this is most commonly seen in small-for-gestational-age infants, large-for-gestational-age infants, infants of diabetic mothers, birth asphyxia, or in rare cases due to primary hyperinsulinism (eg, nesidioblastosis or Beckwith- Wiedemann syndrome). Abdominal distension may compress the thorax and interfere with normal respiration. This may be seen as a result of gastrointestinal pathology (obstruction) or large intra-abdominal mass effect (renal/genitourinary masses, severe ascites). Finally, late preterm (34 - 37 weeks gestation) or even term infants may present with apneic episodes as a cause of cyanosis.
C: Circulation—Cardiac and Circulatory Causes
The hemoglobin circulating in the vasculature plays an important role in oxygenation. Both high and low levels of hemoglobin may lead to cyanosis, although for different reasons. Polycythemia can cause pulmonary hypertension owing to increased viscosity of the blood interfering with pulmonary perfusion. This may be seen in infants of diabetic mothers, delayed clamping of the umbilical cord, chronic fetal hypoxia (eg, placental insufficiency, preeclampsia); in recipient twins of a twinto- twin transfusion syndrome; and in conditions such as trisomy 21. Conversely, severe anemia can cause respiratory distress because inadequate oxygen delivery to tissues can lead to cellular hypoxia. Anemia may be due to hemolytic disease of the newborn, fetal blood loss due to external hemorrhage (placental abruption, umbilical cord rupture), or fetal-maternal hemorrhage, or may be seen in donor twins in twin-to-twin transfusion syndrome.
Abnormalities of the hemoglobin molecule itself may interfere with the normal chemical combination of hemoglobin with oxygen. The most common cause is methemoglobinemia, which results from the oxidation of hemoglobin molecules from the normal ferrous to ferric state. Infants are more susceptible as fetal hemoglobin is more easily oxidized than is adult hemoglobin, and because levels of methemoglobin reductase are relatively low in infants. Methemoglobinemia may result from exposure to oxidants (eg, nitrites, sulfonamides, prilocaine, metoclopropamide) or rarely from congenital deficiency of methemoglobin reductase. The characteristic clinical scenario is a blue-gray–appearing infant without respiratory distress who has decreased oxygen saturation, but normal arterial oxygen tension.
Severe cyanosis is a prominent feature in congenital heart disease associated with diminished pulmonary blood flow or in babies with separate circulations and poor mixing [5]. Diminished pulmonary blood flow is characteristic of tricuspid atresia, pulmonary atresia, pulmonary stenosis, tetralogy of Fallot (TOF), and Ebstein's anomaly. Tetralogy of Fallot represents approximately 10% of cases of congenital heart disease and is one of the most common cyanotic congenital heart lesions presenting in the newborn period. The pulmonary outflow stenosis in TOF tends to be progressive, meaning that clinically significant cyanosis 172 R.H. Steinhorn is present at birth in approximately 25% of infants, but 75% become cyanotic by 1 year of age. In all of these conditions, pulmonary blood flow will initially be dependent on blood directed to the lungs through a patent ductus arteriosus. Therefore, cyanosis worsens at the time of ductal closure and tends to improve rapidly after the ductus is reopened after initiation of prostaglandin E1 (PGE1).
Transposition of the great arteries (TGA) is another relatively common congenital heart lesion that presents with severe cyanosis. The systemic and pulmonary circulations are normally in series with each other, but in complete transposition, the circulations are in parallel. Therefore, deoxygenated systemic venous blood returns to the right atrium, enters the right ventricle, and exits through the aorta. Infants with TGA are dependent on communications between these 2 circuits for mixing. If the ventricular septum is intact, life-threatening cyanosis will develop when the foramen ovale and ductus arteriosus close in the hours or days after birth. Although a patent ductus arteriosus will improve atrial mixing to a variable degree, adequate inter-atrial communication is what allows for adequate mixing and oxygenation. Infants with a large ventricular septal defect may present to the ED after the first few days of life because there is more potential for mixing even as other shunts close.
Cardiac disease associated with complete mixing may be associated with a variable degree of cyanosis. Examples include truncus arteriosus and total anomalous pulmonary venous return, lesions which are characterized by pulmonary overcirculation. Because pulmonary blood flow is normal to increased, cyanosis is usually not as significant and does not respond to PGE1. In fact, measures that increase pulmonary blood flow (PGE1, supplemental oxygen) should be avoided as they may worsen pulmonary overcirculation and thus decrease systemic blood flow. In rare cases, total anomalous pulmonary venous return may be associated with obstruction, which leads to decreased pulmonary blood flow and severe cyanosis.
Persistent pulmonary hypertension of the newborn (PPHN) describes the failure of the normal circulatory transition that occurs after birth [3]. It is characterized by marked pulmonary hypertension that causes hypoxemia and right-to-left extrapulmonary shunting of blood through fetal channels (foramen ovale and ductus arteriosus). The combination of inadequate pulmonary perfusion and extrapulmonary shunting leads to refractory hypoxemia. Persistent pulmonary hypertension often complicates parenchymal lung disease in newborn infants, because pulmonary vessels readily constrict in response to alveolar hypoxia. However, PPHN can also occur idiopathically in the absence of underlying parenchymal disease. In these cases, the syndrome is believed to be the result of an abnormally remodeled vasculature that develops in utero in response to prolonged fetal stress, hypoxia, and/or pulmonary hypertension. PPHN is commonly associated with lung hypoplasia, as seen in congenital diaphragmatic hernia.
Initial Evaluation
The evaluation should systematically assess the infant for airway, pulmonary, and circulatory causes as described above. The history should include an assessment of the pregnancy, labor, and newborn risk factors. A history of maternal diabetes increases the risk of congenital heart disease, as well as polycythemia and hypoglycemia, which may be associated with lethargy and hypoventilation. The presence of oligohydramnios may suggest renal abnormalities associated with hypoplastic lungs, whereas polyhydramnios may suggest airway, esophageal, or neurologic abnormalities. Screening results for cervical colonization of group B streptococcus should be sought, although it is important to realize that infection is possible even if the antenatal culture was negative. Prolonged rupture of membranes may suggest bacterial infection, and a history of a difficult delivery may result in intracranial hemorrhage or phrenic nerve paralysis.
The physical examination should be performed when the infant is appropriately warmed and quieted. The growth characteristics should be noted, as infants who are small or large for gestational age are more prone to polycythemia. The primary focus will be on determining the degree of respiratory distress, as its absence will suggest the presence of congenital heart disease or methemoglobinemia. Respiratory insufficiency due to pulmonary disease is typically characterized by rapid respirations accompanied by retractions and nasal flaring. Neurologic conditions are potential causes of cyanosis because of hypoventilation and may be associated with slow or irregular respirations. It is also important to evaluate the infant's tone and activity, and to assess the infant for periodic breathing and/or apneic spells. The examination may reveal findings of birth trauma, such as an Erb's palsy or stridulous cry.
The cardiac exam should include an assessment of the infant's heart rate, peripheral pulses, and perfusion. Auscultation of the heart should focus on the second heart sound, which will be loud and single (or narrowly split) in pulmonary hypertension, as well as in transposition and pulmonary atresia. The auscultation of heart murmurs is often not helpful: serious lesions such as transposition are not associated with murmurs, and loud murmurs are frequently due to a relatively benign lesion such as a small ventricular septal defect. A notable exception is that a harsh ejection murmur is characteristic of pulmonary stenosis.
As noted above, the oxygen saturation is the percent of hemoglobin that is chemically combined with oxygen, which represents the vast majority of oxygen content in the blood. Pulse oximetry provides excellent noninvasive and continuous assessment of oxygen saturation. New Evaluation and management of the cyanotic neonate 173 generation pulse oximeters appear to improve performance during low perfusion states. It is often useful to obtain simultaneous measurements from the right hand and a foot to determine flow patterns through the ductus arteriosus. As the left subclavian artery may have a preductal or postductal origin from the aorta, it is best not to use the left hand for pulse oximetry monitoring. Although measurement of arterial blood gas oxygen tension is standard practice, the pain of an arterial puncture may produce agitation and changes in ventilation and oxygenation. A venous blood gas may be useful for the assessment of pH and PaCO2, but should not be used to determine oxygenation. In either case, the presence of a significant metabolic acidosis may indicate cardiac failure, sepsis, asphyxia, or metabolic disorders. Many microsampling blood-gas analyzers now include lactate in their measured parameters, providing additional useful information about global perfusion and oxygenation.
A chest radiograph is an integral part of the initial assessment of the cyanotic newborn. The locations of stomach, liver, and heart should be determined to rule out dextrocardia and situs inversus. Examining the lung fields may reveal parenchymal lung disease (remembering that the newborn with pneumonia typically has diffuse rather than focal infiltrates) or lung abnormalities such as cystic adenomatoid malformation. Elevation of either hemidiaphragm by more than 2 intercostal spaces relative to the opposite side suggests diaphragmatic paralysis due to phrenic nerve injury. Hyperinflated lung fields are seen occasionally in lobar emphysema or cystic lesions of the lungs. Decreased pulmonary vascular markings are characteristic of pulmonary stenosis or pulmonary atresia with inadequate ductal shunting and may be seen in infants with idiopathic persistent pulmonary hypertension of the newborn (Figure 2). The size and shape of the heart may yield some clues to the diagnosis: for instance, the “boot shape” heart of TOF, and the “egg on string” appearance of transposition, and the characteristic massive cardiomegaly of Ebstein's anomaly.
An electrocardiogram is useful for the diagnosis of cardiac arrhythmias. However, normal newborns have a predominance of right-sided forces, and moderate right ventricular hypertrophy is a common finding with many types of respiratory and cardiac disease. Therefore, the electrocardiogram is seldom helpful in the evaluation of the infant with congenital heart disease and is often completely normal even in infants with serious disease such as transposition. A notable exception would be the infant with left axis deviation due to left ventricular hypertrophy, which would strongly suggest tricuspid atresia.
Some advocate for the hyperoxia test as a clinical tool to differentiate between pulmonary and cardiac disease in cyanotic infants. The test is based on the principle that in the absence of fixed cardiac shunts, 100% oxygen will increase alveolar PO2, leading to an increase in pulmonary venous and systemic arterial PO2. In cyanotic congenital heart disease (eg, decreased pulmonary blood flow or TGA), little or no rise in PaO2 would be expected after breathing 100% O2. However, the same finding may occur in infants with significant pulmonary hypertension if significant right-to-left shunting persists through extrapulmonary shunts (ductus arteriosus and foramen ovale). Given the wide availability of echocardiography, the hyperoxia test should rarely (if ever) be necessary and should only be considered after discussion with a cardiologist.
Initial Management in the Emergency Department
Severe cyanosis requires urgent supportive therapy while a diagnosis is established. This will include intravenous fluids and withholding of enteral feedings. The infant should be maintained in a thermoneutral environment using a radiant warmer. Hypoglycemia is common in critically ill infants, therefore glucose levels should be monitored and glucose infusions provided to maintain a blood glucose of greater than 55 mg/dL. An airway and assisted ventilation should be considered for infants with respiratory distress, but may be deferred for the comfortable infant. Severe acidosis should be corrected with infusions of sodium bicarbonate, but only after adequate gas exchange has been established. If the infant is less than 10 days old and the umbilical stump is still attached, umbilical venous and arterial lines can frequently be placed Figure 2 Chest x-ray of an infant with idiopathic persistent pulmonary hypertension. Note the clear lung fields with decreased vascularity. Similarly, oligemic lung fields would also be expected in conditions with low pulmonary blood flow, such as pulmonary atresia.
174 R.H. Steinhorn
by experienced practitioners for rapid central access. Hypocalcemia is often associated with cardiac disease and critical illness, and should be corrected based on the ionized calcium. Oxygen should be provided, although there are increasing concerns about the potential risks associated with this therapy [6]. Even brief (30-minute) exposures to extreme hyperoxia are increasingly recognized to increase oxidative stress and potentially damage lung parenchymal and vascular function, even in term infants [7,8]. Therefore, the use of 100% O2 should generally be avoided at the outset. Initiating oxygen therapy with 40% to 60% O2 will allow the caregiver to provide support, assess for improvement, and seek advice from a cardiologist. This point is particularly important if an infant has only a minimal response to oxygen, as this may indicate potential cardiac disease and need for PGE1. In addition, it is important to remember that oxygen may promote ductal closure. This may not be a major concern for lesions that limit pulmonary blood flow, as the pulmonary venous PO2 would not be expected to rise. However, admixture lesions such as hypoplastic left heart syndrome may present with moderate cyanosis. These conditions are dependent on a patent ductus to maintain systemic blood flow. Oxygen may not only promote ductal closure, but may increase pulmonary and decrease systemic blood flow.
In the infant who does not require assisted ventilation, oxygen may be delivered via a head hood or nasal cannula [9]. A head hood is the only method that allows the FiO2 to be determined precisely. The oxygen concentration should be measured by an oxygen analyzer placed near the baby's mouth. Relatively high flows are needed achieve adequate concentrations of oxygen and avoid carbon dioxide accumulation, although humidification is generally not necessary. Although head box oxygen is generally well tolerated, this method limits the infant's mobility, and oxygen concentrations fall quickly when the hood is lifted to provide care to the infant. Therefore, this method is typically not used when prolonged oxygen treatment is required. Oxygen is frequently delivered by a nasal cannula. The disadvantage of this method is that the infant entrains variable amounts of room air around the nasal cannula. Therefore, it cannot provide 100% oxygen, and the oxygen concentration in the hypopharynx (a good proxy for the tracheal concentration) will be much lower than the concentration of oxygen at the cannula inlet. Both the oxygen concentration and the cannula flow rate will be the major factors that will determine the fraction of oxygen actually delivered. Therefore, it is generally better to titrate delivery to achieve the desired oxygen saturation levels, generally 90% to 95% by pulse oximetry. Prostaglandin E1 is clinically effective for infants dependent on ductal patency to maintain pulmonary blood flow or sufficient mixing. It is given intravenously by constant infusion, and the initial dose is typically 0.05 µg/kg per minute. Apnea is a common side effect after initiation of PGE1, and some recommend intubation if the infant will require transport shortly after beginning the infusion. Other common side effects include flushing and diarrhea. There are no absolute contraindications to beginning prostaglandin, although it may worsen the pulmonary edema associated with obstructed total anomalous pulmonary venous return.
Summary
The infant presenting to the ED with cyanosis requires urgent assessment, diagnosis, and initiation of therapy. A systematic, rational approach to the diagnosis of neonatal cyanosis is essential. An understanding of the normal transitional physiology, and how diseases of the airway, lung, and circulatory system may disrupt these processes, will enable the ED practitioner to determine whether the underlying cause is related to airway obstruction, parenchymal disease, hypoventilation due to central nervous system disease or apnea, or due to cardiac disease. Management is based on the clinical diagnosis and attention to hemodynamic stability, judicious oxygen administration, and referral to the appropriate inpatient hospital setting. Although prognosis depends on the diagnosis, it is generally good with prompt recognition and intervention. References
1. Sasidharan P. An approach to diagnosis and management of cyanosis and tachypnea in term infants. Pediatr Clin North Am 2004;51: 999-1021.
2. Lees MH. Cyanosis of the newborn infant. Recognition and clinical evaluation. J Pediatr 1970;77:484-98.
3. Farrow KN, Fliman P, Steinhorn RH. The diseases treated with ECMO: focus on PPHN. Semin Perinatol 2005;29:8-14.
4. Porta NF. Respiratory disorders of the newborn. In: Green T, Franklin W, Tanz R, editors. Pediatrics: Just the Facts. New York: McGraw-Hill; 2005. p. 99-108.
5. Zahka K, Lane J. Approach to the neonate with cardiovascular disease. In: Martin RJ, Fanaroff A, Walsh MC, editors. Fanaroff and Martin's Neonatal-Perinatal Medicine. 8th ed. Philadelphia, PA: Mosby; 2005. p. 1112-20.
6. Saugstad OD, Ramji S, Vento M. Oxygen for newborn resuscitation: How much is enough? Pediatrics 2006;118:789-92.
7. Lakshminrusimha S, Russell JA, Steinhorn RH, et al. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res 2006;59:137-41.
8. Lakshminrusimha S, Russell JA, Steinhorn RH, et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res 2007;62:313-8.
9. Frey B, Shann F. Oxygen administration in infants. Arch Dis Child Fetal Neonatal Ed 2003;88:F84-8.
Pulmonary hypertension
In uncontrolled studies, hyperventilation to achieve carbon dioxide tensions of 20 to 25 torr and an elevation of pH resulted in improvements in oxygenation [95], but such low CO2 levels are associated with a 50% reduction in cerebral blood flow, and hypocarbia has been associated with PVL in preterm infants [96]. Moreover, the technique of hyperventilation was described for primary pulmonary hypertension of the newborn, whereby the pathophysiology was increased pulmonary vascular resistance in the absence of pulmonary parenchymal disease. Applying this technique to parenchymal lung diseases, especially MAS, may be dangerous. Even in the early studies, hyperventilation was associated with an increase in air leak and BPD [97]. Both HFJV and HFOV anecdotally have improved oxygenation in infants who have pulmonary hypertension, but no long-term benefits have been investigated. In contrast, in a randomized trial, ECMO improved survival in infants who have severe PPHN [95].
94] UK Collaborative ECMO Trial Group. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet 1996;348:75–82. [95] Peckham GJ, Fox WW. Physiologic factors affecting pulmonary artery pressure in infants with persistent pulmonary hypertension. J Pediatr 1978;93:1005–10.
Back