



INTRODUCTION
The successful application of CPAP by Gregory in early 1970`s saw a quantum change in the application of neonatal ventilation in respiratory distress syndrome(RDS)1. In India the number of annual deliveries is around 25 million and with estimated rate of prematurity of 7%, about 1.75 million premature babies are expected to be born every year . It is estimated that implementing early intervention strategies can avert more than 60% of neonatal deaths 2. Controversies still exists in the early respiratory management of RDS in Premature infants. Early CPAP therapy has been shown to be successful in some clinical trails in the management of RDS. However there are only few studies reported from our country.
AIM OF OUR STUDY
To estimate the efficacy of early CPAP on,
MATERIALS AND METHOD
Estimation of minimum sample size for this study was based on the efficacy rate reported by HAN etal .Allowing for 30% allowable error on the estimate and with 95% confidence interval . Minimum sample size estimated as 70. We have taken HAN etal study because it is similar to our protocol . Higher allowable error was taken due to the differences in protocols adopted to this study compared to that of earlier studies.
Preterm babies were nursed in a thermoneutral zone using Fischer & paykel warmer with skin temperature set at 36.5 degree Celsius . Intravenous fluids and ionotropes were used when required to keep the mean arterial blood pressure appropriate for the gestation .Oxygen saturations were continuously monitored and kept between 88-90%. Blood gas analysis was done as per protocol . Preterm babies born less than 32 wks were started on Early CPAP soon after birth within 10 - 30 minutes who exihibited Respiratory distress scored by Silverman and Andreson`s score and were observed in our unit as per the protocol .CPAP was delieverd by Nasopharyngeal tube by Babylog 8000 with the flow rate of 6cmH20 in the majority of cases to standardize the management .Bubble CPAP was used with a flow rate of 6cm H20 and binasal prongs when ventilator was not available . Babies who failed CPAP therapy were Intubated & Ventilated and given Surfactant.. Echocardiogram done by paediatric cardiologist was performed as per protocal and significant PDA were treated with Ibuprofen. Neurosonogram was done to detect intraventricular hemorrhage(IVH) ,as per protocol.
Protocol for optimization of CPAP
Babies were started on a nasal CPAP of 5-6 cm H20 and F io2 of 0.4 based on the clinical scoring and titrated with X ray expansion up to 8 spaces and blood gases. Increments in CPAP by 1 cm H20 is done every 15- 20 min to record saturations in the range of above 90% and showing an increasing trend CPAP was increased in a stepwise fashion up to 8 cmH2o& then only Fio2 increments were done by 0 .05% up to a maximum of 0.6
CPAP FAILURE
We defined CPAP failure in those babies who had the any of the following features:
EXCLUSION CRITERIA
We have excluded some of the babies from our study those who had the following features:
Estimation of sample size : Estimation of minimum sample size for this study was based on the efficacy rate reported by HAN etal .Allowing for 30% allowable error on the estimate and with 95% confidence interval . Minimum sample size estimated as 70. Higher allowable error was taken due to the differences in protocols adopted to this study compared to that of earlier studies. RESULTS OF OUR STUDIES
Following are the results which we derived from our studies.In our study, about 16.3% mother had gestational diabetes and 75.5% of mothers had received ante natal steroids (2 full doses - 57.9 %). About 89.8 % of babies were delivered by Caeserian section for fetal indications .
About 83.6 % babies had clinical features of respiratory distress after birth. We delivered CPAP through Ventilator and Bubble CPAP device in 83.7 % and 16.3% respectively.Average duration of CPAP needed was around 7 days (Maximum needed upto 15 days in some babies). Incidence of Patent ductus arteriosus was 17.5%. Sex wise distribution (N= 74)
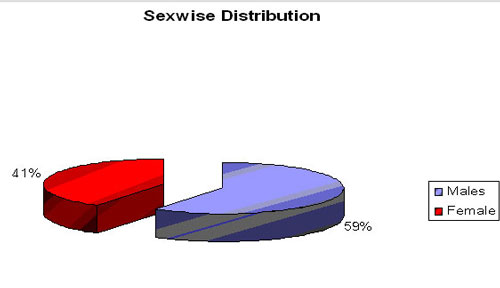
Male : Female ratio - 44:30 (No of cases)
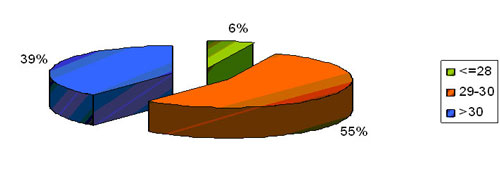
GA wise distribution of RDS ( N=74)
Sex wise distribution (N= 74)
Male : Female ratio - 44:30 (No of cases)
GA wise distribution of RDS ( N=74)
Predominant group was in the gestation of 29-30 weeks followed by
the gestation of more than 30 weeks . In our study babies below 28
weeks represented only a small population .
BirthWeight distribution ( N= 74)
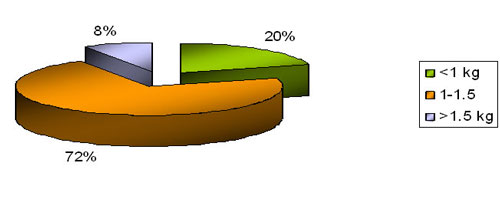
In our study about 2/3 recorded a birth weight more than 1.5kg followed by approximately 1/4 by babies less than 1 kg . OUT COME OF CPAP THERAPY ( n = 74)
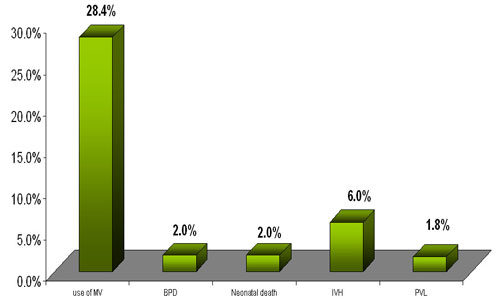
Primary outcome was impressive with only 28.4 % requiring mechanical
ventilation .
Co - morbidities analyzed revealed a 2% incidence of BPD , 6%
incidence of IVH (gradeI and II), 1.8% incidence of PVL and 2%
incidence of neonatal death.
Other Co - Morbidities (N=74))
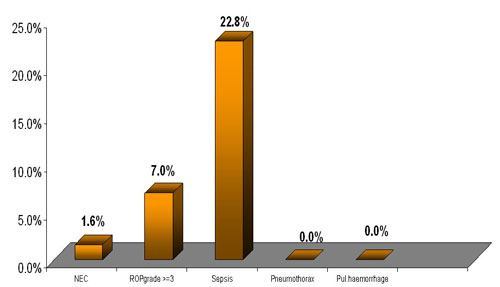
Our study revealed a very significant decreased incidence of NEC
(1.6%) and almost absent airleak and pulmonary hemorrhage . Incidence
of PDA was 7%. Incidence of sepsis was 22.8%.
Complications of CPAP( N= 74)
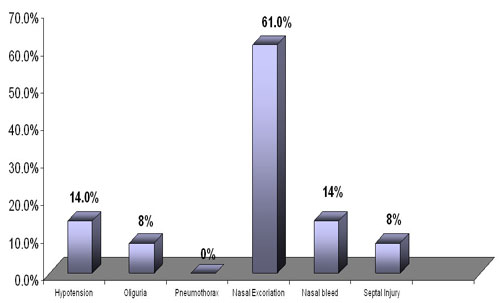
Our study revealed significant complication of nasal excoriation at
61%. But septal injury was only 8%. Nasal bleed was recorded in 14%
.CPAP therapy by our protocol was associated with only 14% incidence
of hypotension and 8% incidence of oliguria .
Gestational Age and survival
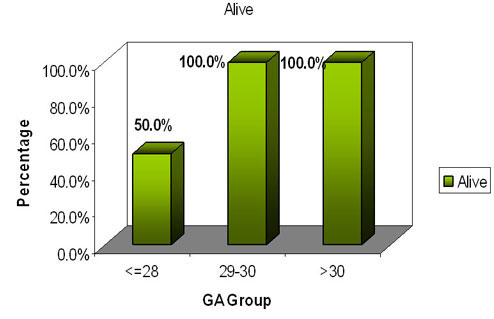
n=4 n=40 n=30
Our study revealed an impressive survival of 100% in the gestational
age group between 28-32 weeks .A survival of 50 % in the gestational
age below 28 weeks was evidenced .
GA wise need for Mechanical ventilation (N=21)
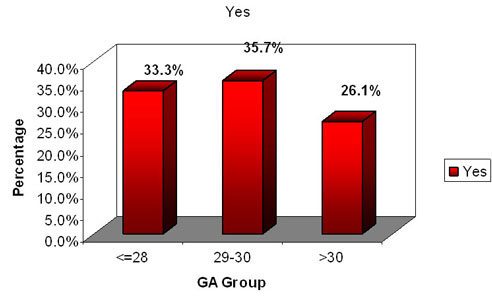
n=7 n=8 n=6
In our study when a analysis of the babies requiring mechanical
ventilation (SIMV and volume guarantee ) approximately 1/3 of each
group required the same
OUTCOME IN VENTILLATED BABIES
In our studies, totally 28.4% babies failed CPAP therapy and they subsequently needed mechanical ventilation. Median duration of ventilator therapy was 4.7 days. Out of ventilated babies two were ventilated for pathological apnea.
On an average we have given only one dose of surfactant to our ventilated babies with only one baby required maximum of two doses.
Outcome in ventilated babies( N=21)
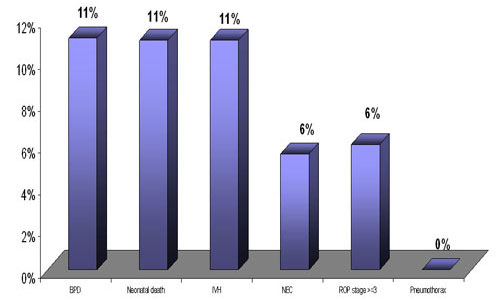
On extended analysis of this subgroup 11% evidenced BPD , IVH (grade I and II). NEC and ROP stage 3 was evidenced in 6 % . Neonatal death was seen in 11%.
Discussion
In the last decade, efforts have been made to improve survivaland long term outcome of preterm newborns. Prenatal steroid administration has reduced the incidence and severity of RDS3. Early identification and treatment of patent ductusarteriosus has reduced lung deterioration from haemodynamicfactors4. Studies on surfactant therapy have helped to establish the best type of surfactant and the optimumtiming of its administration5-9 .The result is a large population of less severely affected preterm infants whocan be treated without MV, thus reducing iatrogenic risks and costs.
In our study we could not create a control group for ethical resaons since CPAP is a standard of practice of primary respiratory assistance in early RDS .
On review of literature we couldnot compare any Indian study with our protocol . we could compare only with two western studies done by,Han et al10 1987 in a presurfactant era and a more recent one by Sandri etal 112005. there were still subtle differences in protocol leading to such a marked difference in primary outcome .
The results of the study show the marked efficacy of CPAP in the management of RDS below 32 weeks with only 28.4 % needing mechanical ventilation . Incidence of airleaks was dramatic by its absence compared to other studies . Incidence of severe grades of IVH was remarkable by its absence compared to other studies . NEC and BPD remained. Very low comparable with Sandri etal . PVL incidence was higher in our study ,as was the incidence of sepsis . Surfactant use after CPAP was comparable with Sandri at 24 %.
Survival was better than Sandri etal at 98% .Outcomes HAN 1987 (n=43)SANDRI 2005(n=115)AIMS 2008(n=74)MV 39.5%12%28.4%Pneumothorax 9.3%2.6%0%BPD 28%1.8%2%IVH (grade 3&4)27.9%( all grades)6%0%PVL Not studied0.9%1.8%NEC9.3%1.7%1.6%ROP -stage34.6%0%7%sepsis16.3%16.5%22.8%Surfactant Not studied22.6%24%Neonatal death 9.3%3.5%2%
CONCLUSION
Early institution of CPAP is a gentle and effective non invasive method in the early management of respiratory distress in premature neonates, which significantly reduced the need for mechanical ventilation and surfactant therapy.It also reduces the incidence of bronchopulmonary dysplasia to a great extent. This modality of therapy is associated with minimum complications compared to other methods.
This study suggests that a trial of early CPAP is not detrimental and may be justified in every case of respiratory distress, provided rescue surfactant therapy is given if the infant needs to be intubated and ventilated.
Early use of CPAP will be a low cost, simple and non invasive option for a country like India,where most places cannot provide invasive ventilation and Surfactant.
It was impressive to note that the number of doses of Surfactant needed were very less in those babies who failed early CPAP therapy. So,whether the use of early CPAP helps reducing in the number of doses of rescue surfactant is a question and to be analyzed by more control trials.
Based on this study, randomized controlled trial are warranted for further analysis regarding the efficacy of CPAP
LIMITATIONS OF OUR STUDY
Our study is not a randomized controlled trial. Comparisons were could only be made by taking populations of a different race (from western studies). Proportion of babies below 28wks of GA is low compared to other studies. Bibilography
| Recipient of the "Certificate of exemplary performance" from the Head of the Department of Perinatal Medicine, Westmead Hospital, Sydney. |
 |
| Recipient of the "Rajiv Gandhi Shiromani Award" of the National Integration and Economic Council, New Delhi, for "Excellence in Neonatology" |
| More [+] |


